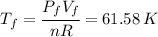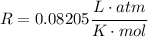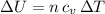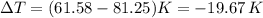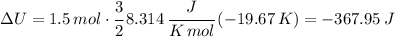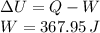Physics, 18.09.2019 20:30 jrsavala559p9969g
An insulated vessel contains 1.5 moles of argon at 2 atm. the gas initially occupies a volume of 5 l. as a result of the adiabatic expansion the pressure of the gas is reduced to 1 atm. (a) find the volume and temperature of the final state. (b) find the temperature of the gas in the initial state. (c) find the work done by the gas in the process. (d) find the change in the internal energy of the gas in the process.

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:30
Ann walks 80 meters on a straight line 33 ∘ north of east starting at point 1. draw ann's path. represent ann's walk with a vector of length 80 meters. draw the vector starting at point 1. the length given in the display is in meters.
Answers: 1

Physics, 22.06.2019 02:10
"an open tank has the shape of a right circular cone. the tank is 6 feet across the top and 5 feet high. how much work is done in emptying the tank by pumping the water over the top edge? note: the density of water is 62.4 lbs per cubic foot."
Answers: 2

Physics, 22.06.2019 04:30
You are traveling in a car going at a constant speed of 100km/h down a long, straight highway. you pass another car going in the same direction which is traveling at a constant speed of 80km/h. as measured from your car's reference frame this other car is traveling at −20km/h. what is the acceleration of your car as measured from the other car's reference frame? what is the acceleration of the other car as measured from your car's reference frame?
Answers: 2

Physics, 22.06.2019 11:20
More solar radiation is absorbed by earth’s surface than by
Answers: 1
You know the right answer?
An insulated vessel contains 1.5 moles of argon at 2 atm. the gas initially occupies a volume of 5 l...
Questions

Business, 09.09.2021 07:20

Chemistry, 09.09.2021 07:20

Chemistry, 09.09.2021 07:20






History, 09.09.2021 07:20





Mathematics, 09.09.2021 07:20


French, 09.09.2021 07:20



Mathematics, 09.09.2021 07:20

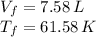
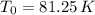
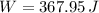
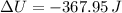
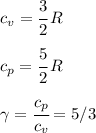
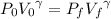
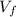 as follows:
as follows: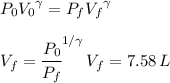
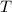 :
: