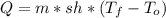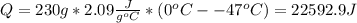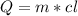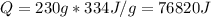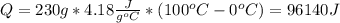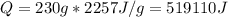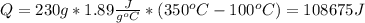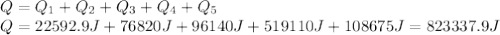Physics, 17.09.2019 23:20 7thaohstudent
Water has the following thermodynamic constants: (1) specific heat liquid = 4.18 j/g °c, solid = 2.09 j/g °c, gas = 1.89 j/g °c, (2) heat of fusion = 334 j/g, and (3) heat of vaporization = 2257 j/g. for a sample of water at 1.0 atm of pressure, mass = 230 g at an initial temperature of -47 °c and a final temperature of 350 °c, answer the following questions: (1) how much heat is required to warm the solid sample to its melting point? j (2) how much heat is required to melt the sample? j (3) how much heat is required to warm the liquid sample to its boiling point? j (4) how much heat is required to vaporize the sample? j (5) how much heat is required to warm the gaseous sample to its final temperature? j and finally, (6) how much heat is required for the entire process to occur

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:30
Explain the difference between each pair of concepts. a. frequency and relative frequency b. percentage and relative frequency a. select the correct choice below. a. frequency is the total number of observations in a data set. relative frequency is the number of times a particular distinct value occurs. b. frequency is the number of times a particular distinct value occurs. relative frequency is the ratio of the frequency of a value to the total number of observations. c. frequency is the total number of observations in a data set. relative frequency is the ratio of the number of times a particular distinct value occurs to the frequency. d. frequency is the number of times a particular distinct value occurs. relative frequency is the ratio of the frequency of two different values.
Answers: 3

Physics, 22.06.2019 11:30
What is the name for the remnant of an asymptotic giant that has lost its shells? black dwarf white dwarf yellow giant black hole
Answers: 3

Physics, 22.06.2019 18:00
Aprisoner is forced to go into one of three rooms, but he can choose which room. the first room is ablaze with fire. the second one is rigged with explosives that will go off as soon as he enters. the third contains a pair of lions who haven't eaten in years. which room should he choose to survive?
Answers: 2

Physics, 22.06.2019 23:30
The photo above shows oil and vinegar in a pitcher. the top make a claim about about the density of the vinegar compared to the density of the oil summarize the evidence to support the claim and explain your reasoning
Answers: 3
You know the right answer?
Water has the following thermodynamic constants: (1) specific heat liquid = 4.18 j/g °c, solid = 2....
Questions

Mathematics, 08.12.2021 05:20

Mathematics, 08.12.2021 05:20

Mathematics, 08.12.2021 05:20


Mathematics, 08.12.2021 05:20

Mathematics, 08.12.2021 05:20



Mathematics, 08.12.2021 05:20



French, 08.12.2021 05:20



Social Studies, 08.12.2021 05:20

Mathematics, 08.12.2021 05:20


Physics, 08.12.2021 05:20