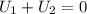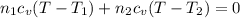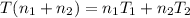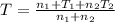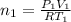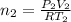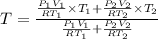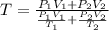Physics, 14.09.2019 07:20 jamalnellum56
Arigid adiabatic container is divided into two parts containing n1 and n2 mole of ideal gases respectively, by a movable and thermally conducting wall. their pressure and volume are p1, v1 for part 1 and p2, v2 for part 2 respectively. find the final pressure p and temperature t after the two gas reaches equilibrium. assume the constant volume specific heats of the two gas are the same.

Answers: 2


Another question on Physics

Physics, 22.06.2019 06:00
Using a pedometer, you walk 3000 steps in 20 minutes, so your speed is 150 steps/min. each of your steps is 0.7 m long. what is your speed? 150 steps/min=/s=/h
Answers: 1

Physics, 22.06.2019 06:30
2kg of refrigerant 134a undergoes a polytropic process in a piston-cylinder assembly from an initial state of saturated vapor at 2 bar to a final state of 12 bar, 80 degree c. a)determine the work for the process in kj. b)sketch the process on a p-v diagram.
Answers: 2

Physics, 22.06.2019 10:30
Find the magnetic field a distance r from the center of a long wire that has radius a and carries a uniform current per unit area j in the positive z direction.
Answers: 2

Physics, 22.06.2019 15:10
Auniform crate c with mass mc is being transported to the left by a forklift with a constant speed v1. what is the magnitude of the angular momentum of the crate about point a, that is, the point of contact between the front tire of the forklift and the ground
Answers: 3
You know the right answer?
Arigid adiabatic container is divided into two parts containing n1 and n2 mole of ideal gases respec...
Questions


Chemistry, 02.09.2021 01:00

Mathematics, 02.09.2021 01:00

Mathematics, 02.09.2021 01:00




Chemistry, 02.09.2021 01:00

Mathematics, 02.09.2021 01:00

Spanish, 02.09.2021 01:00

English, 02.09.2021 01:00

Advanced Placement (AP), 02.09.2021 01:00

Mathematics, 02.09.2021 01:00

Mathematics, 02.09.2021 01:00





Mathematics, 02.09.2021 01:00

Mathematics, 02.09.2021 01:00

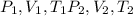
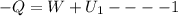
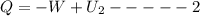
 change in internal Energy of gas
change in internal Energy of gas