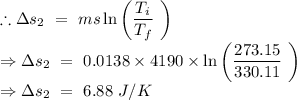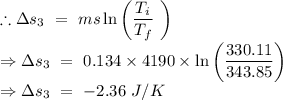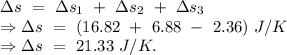Physics, 14.09.2019 02:30 kelseychristian24
An insulated thermos contains 134 g of water at 70.7°c. you put in a 13.8 g ice cube at 0.00°c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg*k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?

Answers: 2


Another question on Physics

Physics, 21.06.2019 18:00
Abaseball has a mass of 0.145 kg and approaches a bat at 40.0 m/s. after it is hit, the ball leaves the bat at 50.0 m/s directly back. find the impulse of the bat on the ball. 7.25 kg*m/s 13.1 kg*m/s 5.80 kg*m/s 1.45 kg*m/s
Answers: 2

Physics, 21.06.2019 22:00
In 1980, alfa romeo introduced the first system to smooth a rough idle caused by ignition and camshaft timing changes.
Answers: 1

Physics, 21.06.2019 22:30
How many molecules are present in 3 mols of silicon dioxide (sio2)?
Answers: 1

Physics, 22.06.2019 11:30
Water is siphoned from a large tank and discharges into the atmosphere through a 50-mm diameter tube. the end of the tube is b = 2.1 m below the tank bottom which is a = 7.4 m deep, and viscous effects are negligible. determine the maximum height h over which the water can be siphoned without cavitation occurring. atmospheric pressure is 101.4 kpa, and the water vapor pressure is 1.79 kpa (absolute)
Answers: 3
You know the right answer?
An insulated thermos contains 134 g of water at 70.7°c. you put in a 13.8 g ice cube at 0.00°c to fo...
Questions

Mathematics, 15.10.2019 09:10


Biology, 15.10.2019 09:10


Biology, 15.10.2019 09:10



Geography, 15.10.2019 09:10





Biology, 15.10.2019 09:10

Computers and Technology, 15.10.2019 09:10


History, 15.10.2019 09:10



Chemistry, 15.10.2019 09:10

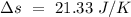
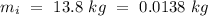 Temperature of the ice =
Temperature of the ice = 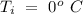 Mass of the original water =
Mass of the original water = 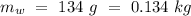 Temperature of the original water =
Temperature of the original water = 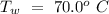 Specific heat of water =
Specific heat of water = 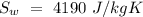 Latent heat of fusion of ice =
Latent heat of fusion of ice = 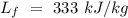
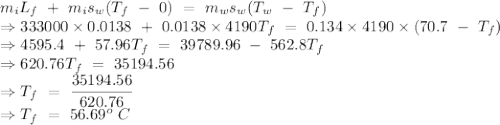
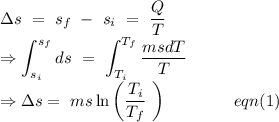
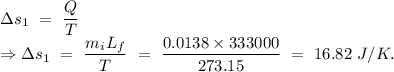
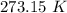 to water 330.11 K water.
to water 330.11 K water.