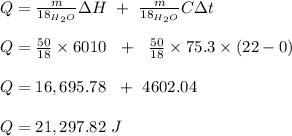Physics, 05.09.2019 02:30 Seaisnowblue
The heat of fusion of water is 6.01 kj/mol. the heat capacity of liquid water is 75.3 j/mol · k. the conversion of 50.0 g of ice at 0.00°c to liquid water at 22.0°c requires kj of heat.

Answers: 1


Another question on Physics

Physics, 21.06.2019 13:30
An air bubble of volume 20 cm³ is at the bottom of a lake 40 m deep, where the temperature is 4.0°c. the bubble rises to the surface, which is at a temperature of 20°c.take the temperature of the bubble’s air to be the same as that of the surrounding water. just as the bubble reaches the surface, what is its volume?
Answers: 1

Physics, 22.06.2019 00:40
Aballet student who learns with the of his instructor is demonstrating learning.
Answers: 3

Physics, 22.06.2019 04:00
Assume similar data for the motion of the blood in your aorta. estimate how many beats of the heart it will take the blood to get from your heart to your brain. (assume that the distance from your heart to your brain is 30 cm)
Answers: 2

Physics, 22.06.2019 10:30
Air is to be preheated by hot exhaust gases in a cross-flow heat exchanger before it enters the furnace. air enters the heat exchanger at 95 kpa and 20°c at a rate of 0.6 m^3/s. the combustion gases (cp = 1.10 kj/kg°c) enter at 160°c at a rate of 0.95 kg/s and leave at 95°c. determine the rate of heat transfer to the air and its outlet temperature.
Answers: 2
You know the right answer?
The heat of fusion of water is 6.01 kj/mol. the heat capacity of liquid water is 75.3 j/mol · k. the...
Questions




Biology, 17.10.2019 15:30


Mathematics, 17.10.2019 15:30

Biology, 17.10.2019 15:30





History, 17.10.2019 15:30








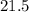 kJ
kJ 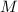 = mass of 1 mole of water = 18 g
= mass of 1 mole of water = 18 g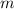 = given mass of water = 50 g
= given mass of water = 50 g 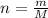
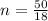
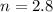
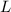 = Heat of fusion of water = 6.01 kJ/mol = 6010 J/mol
= Heat of fusion of water = 6.01 kJ/mol = 6010 J/mol = specific heat of water = 75.3 J/mol
= specific heat of water = 75.3 J/mol = Change in temperature = 22.0 °C
= Change in temperature = 22.0 °C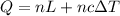
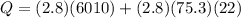
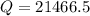 J
J 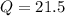 kJ
kJ