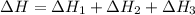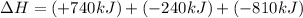Physics, 04.09.2019 17:20 julialombardo53
Given the thermochemical equations x2+3y2⟶2xy3δh1=−370 kj x2+2z2⟶2xz2δh2=−120 kj 2y2+z2⟶2y2zδh3=−270 kj calculate the change in enthalpy for the reaction. 4xy3+7z2⟶6y2z+4xz2

Answers: 1


Another question on Physics

Physics, 22.06.2019 00:30
Which of the following elements is in the same period as phosphorus? a. carbon c. nitrogen b. magnesium d. oxygen select the best answer from the choices provided a b c d
Answers: 1

Physics, 22.06.2019 06:30
How much force was applied to an object if was moved 2 meters and the work done on the object was 8 joules? a. 0.25 n b. 4 n c. 6 n d. 16 n
Answers: 1

Physics, 22.06.2019 16:30
Using gravity, an elephant can pull a mouse towards it from the ground. true or false explain why. will give brainliest if answered fast and the best explanation
Answers: 2

Physics, 22.06.2019 17:20
In a system with only a single force acting upon a body, what is the relationship between the change in kinetic energy and the work done by the force? answers: work is equal to the change in kinetic energy.work depends on the square of the change in potential energy.work is equal to the negative of the change in kinetic energy.work is equal to the square of the change in kinetic energy
Answers: 2
You know the right answer?
Given the thermochemical equations x2+3y2⟶2xy3δh1=−370 kj x2+2z2⟶2xz2δh2=−120 kj 2y2+z2⟶2y2zδh3=−270...
Questions






English, 04.12.2021 01:00


Mathematics, 04.12.2021 01:00


Computers and Technology, 04.12.2021 01:00



Mathematics, 04.12.2021 01:00



Social Studies, 04.12.2021 01:00

Mathematics, 04.12.2021 01:00



Biology, 04.12.2021 01:00

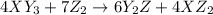
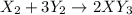
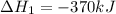
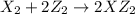
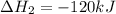
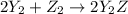
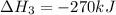
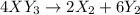
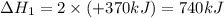
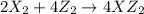
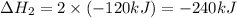
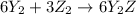
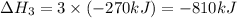
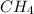 will be,
will be,