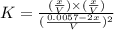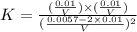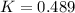Physics, 31.08.2019 03:10 codycollier
200 cm3 of hydrogen iodide gas (hi) is allowed to dissociate and come to equilibrium. the mixture at equilibrium contained 350 cm3 of gaseous iodine. calculate k, for the reaction 2hi(g)h(g)+ ig) (assume that 1 mol of a gas has a volume of 35 000 cm under the conditions in this experiment.)

Answers: 3


Another question on Physics

Physics, 22.06.2019 04:30
The graph describes the motion of an object. the object moves with from a to b. it from b to c. it moves with from c to d.
Answers: 1

Physics, 22.06.2019 14:30
Which of the following bonds would be most polar? a. c-i b. c-br c. c-cl d. c-f e. c-o
Answers: 1

Physics, 22.06.2019 16:40
The force needed to overcome static friction is usually less than that needed to overcome kinetic friction.true or false?
Answers: 1

You know the right answer?
200 cm3 of hydrogen iodide gas (hi) is allowed to dissociate and come to equilibrium. the mixture at...
Questions

Social Studies, 05.06.2020 09:58





Mathematics, 05.06.2020 09:58


Mathematics, 05.06.2020 09:58


Mathematics, 05.06.2020 09:58



Social Studies, 05.06.2020 09:58




Mathematics, 05.06.2020 09:58


History, 05.06.2020 09:58

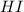 =
= 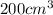
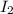 at equilibrium =
at equilibrium = 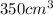
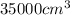
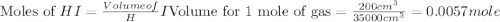
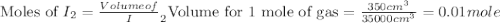
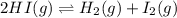
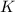 will be,
will be,![K=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0213/8500/3a600.png)