
The energy of the electron in hydrogen atom can be shown to be given by en = -13.6/n^2 ev, where n represents the principal quantum number. imagine a situation where the electron makes a transition from n = 3 to n = 1 state. where does the photon emitted in this transition lie in the electromagnetic spectrum? (a) visible region (b) infra-red region (c) ultra-violet region (d) x-ray region

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:00
How is acceleration calculated? a. initial velocity - final velocity / timeb. initial velocity + final velocity / timec. final velocity - initial velocity / timed. final velocity / timele select the best answer from the choices provided0oood00save and exitnextmark this and return
Answers: 1

Physics, 22.06.2019 00:30
Consider an ordinary, helium-filled party balloon with a volume of 2.2 ft3. the lifting force on the balloon due to the outside air is the net resultant of the pressure distribution exerted on the exterior surface of the balloon. using this fact, we can derive archimedes’ principle, namely that the upward force on the balloon is equal to the weight of the air displaced by the balloon. assuming that the balloon is at sea level, where the air density is 0.002377 slug/ft3, calculate the maximum weight that can be lifted by the balloon. note: the molecular weight of air is 28.8 and that of helium is 4.
Answers: 2


Physics, 22.06.2019 10:00
Asap and show ! a 14 kg rock starting from rest free falls through a distance of 5.0 m with no air resistance. find the momentum change of the rock caused by its fall and the resulting change in the magnitude of earths velocity. earth mass is 6.0 * 10^24 kg. show your work assuming the rock earth system is closed.
Answers: 2
You know the right answer?
The energy of the electron in hydrogen atom can be shown to be given by en = -13.6/n^2 ev, where n r...
Questions

Mathematics, 26.06.2019 23:30

Physics, 26.06.2019 23:30


Health, 26.06.2019 23:30

Mathematics, 26.06.2019 23:30



Mathematics, 26.06.2019 23:30

Social Studies, 26.06.2019 23:30


English, 26.06.2019 23:30

English, 26.06.2019 23:30

Biology, 26.06.2019 23:30


Mathematics, 26.06.2019 23:30





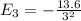
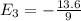 = -1.511 eV
= -1.511 eV 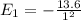
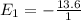 = 13.6 eV
= 13.6 eV

