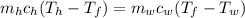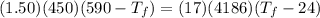Physics, 01.08.2019 00:10 batman48000
A1.50-kg iron horseshoe initially at 590°c is dropped into a bucket containing 17.0 kg of water at 24.0°c. what is the final temperature of the water–horseshoe system? ignore the heat capacity of the container and assume a negligible amount of water boils away.

Answers: 3


Another question on Physics


Physics, 22.06.2019 11:30
In order of decreasing light-transmitting capabilities of materials, which is the correct sequence? a. transparent -> translucent -> opaque b. opaque -> transparent -> translucent c. opaque -> translucent -> transparent d. translucent -> transparent -> opaque
Answers: 1

Physics, 22.06.2019 18:50
An insulated thermos contains 148 g of water at 72.7 ˚c. you put in a 11.7 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?
Answers: 2

Physics, 22.06.2019 23:00
Which scientist was the first to propose the heliocentric model of the universe? a. aristotle b. isaac newton c. galileo galilei d. nicolaus copernicus
Answers: 1
You know the right answer?
A1.50-kg iron horseshoe initially at 590°c is dropped into a bucket containing 17.0 kg of water at 2...
Questions






English, 26.11.2021 02:40



Spanish, 26.11.2021 02:40











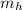 = mass of iron horseshoe = 1.50 kg
= mass of iron horseshoe = 1.50 kg 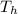 = Temperature of iron horseshoe initially = 590 °C
= Temperature of iron horseshoe initially = 590 °C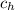 = specific heat of iron = 450 J/(kg °C)
= specific heat of iron = 450 J/(kg °C)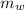 = mass of water = 17 kg
= mass of water = 17 kg 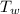 = Temperature of water initially = 24.0 °C
= Temperature of water initially = 24.0 °C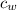 = specific heat of water = 4186 J/(kg °C)
= specific heat of water = 4186 J/(kg °C)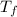 = Final equilibrium Temperature
= Final equilibrium Temperature