
Physics, 31.07.2019 21:10 lightning1157blaze
A0.468 g sample of pentane, c 5h 12, was burned in a bomb calorimeter. the temperature of the calorimeter and 1.00 kg of water in it rose from 20.45 °c to 23.65 °c. the heat capacity of the calorimeter by itself is 2.21 kj/°c and the specific heat capacity of water is 4.184 j/g.°c what is the heat of combustion per mole of pentane?

Answers: 2


Another question on Physics

Physics, 21.06.2019 17:50
Frost wedging occurs when water seeps into a crack in a rock, freezes, and causes the crack in the rock to widen. t or f
Answers: 1

Physics, 22.06.2019 06:10
Africtionless piston-cylinder contains 50 l of saturated liquid r-134a. the piston has a mass and an area resulting in an applied pressure of 500 kpa on the refrigerant. the refrigerant is now heated until its temperature rises to 70∘c. calculate the work (in kj) done during this process.
Answers: 3

Physics, 22.06.2019 10:00
One object has a mass of 1 kg and another object has a mass of 3 kg. if the speeds are the same, which of the following is true about their kinetic energy?
Answers: 2

Physics, 22.06.2019 12:00
An architect plans to use solar energy to heat the next house he designs. what principle of absorption and infrared energy can be applied to the design of the new house? how could she apply to those principals?
Answers: 2
You know the right answer?
A0.468 g sample of pentane, c 5h 12, was burned in a bomb calorimeter. the temperature of the calori...
Questions

World Languages, 18.09.2019 15:20

Chemistry, 18.09.2019 15:20



Mathematics, 18.09.2019 15:20

English, 18.09.2019 15:20

Chemistry, 18.09.2019 15:20

History, 18.09.2019 15:20




SAT, 18.09.2019 15:20

Biology, 18.09.2019 15:20


Mathematics, 18.09.2019 15:20

Mathematics, 18.09.2019 15:20

Computers and Technology, 18.09.2019 15:20

Mathematics, 18.09.2019 15:20


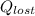 per mole of pentane = 3157.53 kJ/mol
per mole of pentane = 3157.53 kJ/mol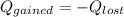


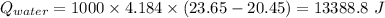


 (Here negative sign depicts the release of the heat)
(Here negative sign depicts the release of the heat)

