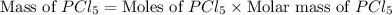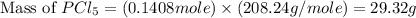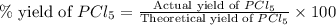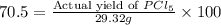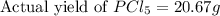Phosphorous reacts with chlorine gas to produce phosphorous pentachloride. calculate the mass of product produced when 25.0 g of phosphorous reacts with 25.0 grams of chlorine. calculate the mass of product produced if the reaction occurred with a 70.5 percent yield.

Answers: 1


Another question on Physics


Physics, 22.06.2019 05:30
Which of the choices below is one of the primary gases found in the atmosphere? a. helium b. carbon dioxide c. nitrogen d. argon
Answers: 2

Physics, 22.06.2019 11:30
Achlorine atom has 17 protons and 18 neutrons. what is its mass number? what is its atomic number?
Answers: 2

Physics, 22.06.2019 14:10
Amachinist turns the power on to a grinding wheel, at rest, at time t = 0 s. the wheel accelerates uniformly for 10 s and reaches the operating angular velocity of 96 rad/s. the wheel is run at that angular velocity for 40 s and then power is shut off. the wheel slows down uniformly at 1.5 rad/s2 until the wheel stops. in this situation, the time interval of deceleration is closest to:
Answers: 3
You know the right answer?
Phosphorous reacts with chlorine gas to produce phosphorous pentachloride. calculate the mass of pro...
Questions

Mathematics, 16.04.2020 21:14

History, 16.04.2020 21:14

Mathematics, 16.04.2020 21:14









Computers and Technology, 16.04.2020 21:14



Mathematics, 16.04.2020 21:14


Mathematics, 16.04.2020 21:14

Mathematics, 16.04.2020 21:14

English, 16.04.2020 21:14

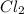 = 25 g
= 25 g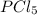 = 208.24 g/mole
= 208.24 g/mole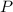 and
and 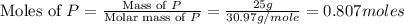
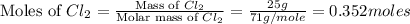
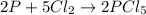
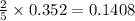 moles of
moles of