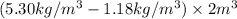A2-m3 rigid tank initially contains air whose density is 1.18 kg/m3 . the tank is connected to a high-pressure supply line through a valve. the valve is opened, and air is allowed to enter the tank until the density in the tank rises to 5.30 kg/m3 . determine the mass of air that has entered the tank?

Answers: 1


Another question on Physics

Physics, 22.06.2019 06:00
An object moves from position +34m to the position -15m in 15 seconds. what is the total displacement? what is the total velocity?
Answers: 3

Physics, 22.06.2019 17:00
In the future, people will only enjoy one sport: electrodisc. in this sport, you gain points when you cause metallic discs hovering on a field to exchange charge. you are an electrodisc player playing the popular four disc variant. the disks have charges of qa = −8.0 µc, qb = −2.0 µc, qc = +5.0 µc, and qd = +12.0 µc. (1) you bring two disks together and then separate them. you measure the resulting charge of these two disks and find that it is +5.0 µc per disk. which two disks did you bring together? (a) a and b (b) a and c (c)a and d (d)b and c(e) b and d (f) c and d. (2) you bring three disks together and then separate them. you measure the resulting charge of these three disks and find that it is +3.0 µc per disk. which three disks did you bring together? a, b, and c (a) a, b, and d (c) a, c, and d (d) b, c, and d. (3) given the resulting charge of each disk measured in (b) is +3.0 µc, how many electrons would you need to add to a disk of this charge to electrically neutralize it? electrons
Answers: 3

Physics, 22.06.2019 23:00
Awelder using a tank of volume 7.50×10^-2 m^3 fills it with oxygen (with a molar mass of 32.0 g/mol ) at a gauge pressure of 3.30×10^5 pa and temperature of 37.1 ∘c. the tank has a small leak, and in time some of the oxygen leaks out. on a day when the temperature is 23.1 ∘c, the gauge pressure of the oxygen in the tank is 2.00×10^5 pa . a) find the initial mass of oxygen. b) find the mass of oxygen that has leaked out.
Answers: 3

Physics, 23.06.2019 01:30
The flame produced by the burner of a gas (propane) grill is a pale blue color when enough air mixes with the propane (c3h8) to burn it completely. for every gram of propane that flows through the burner, what volume of air is needed to burn it completely? assume that the temperature of the burner is 195.0°c, the pressure is 1.05 atm, and the mole fraction of o2 in air is 0.210.
Answers: 2
You know the right answer?
A2-m3 rigid tank initially contains air whose density is 1.18 kg/m3 . the tank is connected to a hig...
Questions


Mathematics, 02.04.2020 21:35


Mathematics, 02.04.2020 21:35

English, 02.04.2020 21:35


Mathematics, 02.04.2020 21:35




Computers and Technology, 02.04.2020 21:35






Mathematics, 02.04.2020 21:36



Mathematics, 02.04.2020 21:36

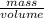
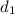 is 1.18
is 1.18 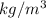 ,
, 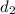 is 5.30
is 5.30 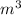 .
.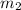 -
- 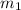 and it will be calculated as follows.
and it will be calculated as follows.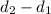 =
= 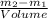
 =
=