
A31.15−g stainless steel ball bearing at 103.27°c is placed in a constant-pressure calorimeter containing 110.3 g of water at 22.59°c. if the specific heat of the ball bearing is 0.474 j / (g · °c), calculate the final temperature of both the water and steel when they equilibrate. assume the calorimeter to have negligible heat capacity.

Answers: 1


Another question on Physics

Physics, 21.06.2019 20:20
An asteroid is discovered in a nearly circular orbit around the sun, with an orbital radius that is 2.47 times earth's. what is the asteroid's orbital period, its "year," in terms of earth years?
Answers: 1

Physics, 22.06.2019 01:30
The passing of heat through a material while the material itself stays in place. a. radiation b. conduction c. convection
Answers: 2

Physics, 22.06.2019 03:00
If a spring has a k value of 100 newtons per meter and it is stretched 0.50 meters, what is the restoring force of the spring?
Answers: 2

Physics, 22.06.2019 13:00
The magnitude of the amount of energy released by burning a fuel source, measured in energy per unit mass, is called its fuel value. note that the fuel value is the negative of the isobaric specific heat of combustion for the fuel. if all the energy obtained from burning 1.23 pounds of butane with a fuel value of 10.85 kcal/g is used to heat 128.0 kg of water at an initial temperature of 18.3 °c, what is the final temperature? note that 1 lb = 453.6 g.
Answers: 3
You know the right answer?
A31.15−g stainless steel ball bearing at 103.27°c is placed in a constant-pressure calorimeter conta...
Questions

Social Studies, 20.07.2019 05:30


Mathematics, 20.07.2019 05:30

Mathematics, 20.07.2019 05:30

Biology, 20.07.2019 05:30

Biology, 20.07.2019 05:30

Social Studies, 20.07.2019 05:30

English, 20.07.2019 05:30

History, 20.07.2019 05:30

Biology, 20.07.2019 05:30

History, 20.07.2019 05:30

History, 20.07.2019 05:30





Chemistry, 20.07.2019 05:30


Health, 20.07.2019 05:30

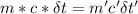
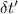 = change in temperature of water = t-22.59
= change in temperature of water = t-22.59

