Physics, 20.07.2019 04:20 sequoyaburke
To treat a burn on his hand, a person decides to place an ice cube on the burned skin. the mass of the ice cube is 16.4 g, and its initial temperature is −13.6 ∘c. the water resulting from the melted ice reaches the temperature of his skin, 31.7 ∘c. how much heat is absorbed by the ice cube and resulting water? assume that all of the water remains in the hand. constants for water can be found in this

Answers: 2


Another question on Physics

Physics, 21.06.2019 20:30
Protons and neutrons are found within the nucleus of an atom
Answers: 2

Physics, 22.06.2019 06:00
The magnetic field inside a superconducting solenoid is 4.50 t. the solenoid has an inner diameter of 6.20 cm and a length of 26.0 cm. determine (a) the magnetic energy density in the field and (b) the energy stored in the magnetic field within the solenoid.
Answers: 2

Physics, 22.06.2019 09:00
Sobre transformações cíclicas, são feitas algumas afirmações, determine quais estão corretas. i – a variação de energia interna em uma transformação cíclica é nula; ii – o trabalho, em uma transformação cíclica, é sempre positivo; iii – ao completar um ciclo, o gás tem a mesma temperatura com a qual começou a transformação; iv – uma transformação cíclica é, obrigatoriamente, composta de transformações conhecidas, como adiabática, isocórica, etc: a) i e iii b) i e ii c) ii e iii d) i e iv e) iii e iv
Answers: 1

Physics, 22.06.2019 09:00
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 1
You know the right answer?
To treat a burn on his hand, a person decides to place an ice cube on the burned skin. the mass of t...
Questions

Mathematics, 03.12.2019 11:31

Social Studies, 03.12.2019 11:31

History, 03.12.2019 11:31

History, 03.12.2019 11:31




Mathematics, 03.12.2019 11:31




Mathematics, 03.12.2019 11:31

English, 03.12.2019 11:31




Mathematics, 03.12.2019 11:31

Health, 03.12.2019 11:31


Biology, 03.12.2019 11:31

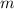 = mass of ice = 16.4 g
= mass of ice = 16.4 g 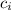 = specific heat of ice = 2.087 J/(g °C)
= specific heat of ice = 2.087 J/(g °C)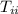 = initial temperature of ice = - 13.5 °C
= initial temperature of ice = - 13.5 °C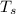 = Skin temperature = 31.7 °C
= Skin temperature = 31.7 °C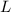 = latent heat of fusion of ice to water = 334 J/g
= latent heat of fusion of ice to water = 334 J/g 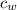 = specific heat of water = 4.186 J/(g °C)
= specific heat of water = 4.186 J/(g °C)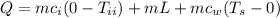
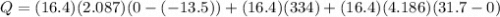
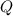 = 8115.88 J
= 8115.88 J