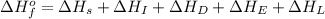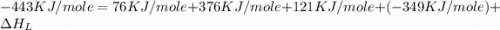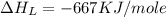Physics, 20.07.2019 04:10 gunaranjan09
The overall energy involved in the formation of cscl from cs(s) and cl2(g) is −443 kj/mol. given the following information: heat of sublimation for cs is +76 kj/mol, bond dissociation energy for 12cl2 is +121 kj/mol, ei1 for cs is +376 kj/mol, and eea for cl(g) is −349 kj/mol. what is the magnitude of the lattice energy for cscl?

Answers: 2


Another question on Physics

Physics, 21.06.2019 14:00
How much heat is required to convert 0.3 kg of ice at 0°c to water at the same temperature
Answers: 1

Physics, 22.06.2019 12:10
Ablock having mass m slides down an inclined plane. the force of friction between the block and the inclined plane is f, the block's weight is m g, and the normal force is n. (a) draw a free – body force diagram showing the forces acting on the block. (b) write down all relevant newton’s equations for a given situation.
Answers: 1

Physics, 22.06.2019 16:00
The field between two charged parallel plates is kept constant. if the two plates are brought closer together, the potential difference between the two plates either a) decrease b) does not change c) increase?
Answers: 3

Physics, 23.06.2019 00:00
Does the distance travelled by an oscillating mass between times t=0 and t2 equal the displacement of the particle over the same time period if t2> t/2? hint: consider a sine wave starting at t1=0, then consider a closing wave starting at t1=0, for your explanation.
Answers: 1
You know the right answer?
The overall energy involved in the formation of cscl from cs(s) and cl2(g) is −443 kj/mol. given the...
Questions

Health, 04.01.2020 06:31

Mathematics, 04.01.2020 06:31


Mathematics, 04.01.2020 06:31

Mathematics, 04.01.2020 06:31

Geography, 04.01.2020 06:31

Mathematics, 04.01.2020 06:31


Mathematics, 04.01.2020 06:31

Social Studies, 04.01.2020 06:31

History, 04.01.2020 06:31



Mathematics, 04.01.2020 06:31


Mathematics, 04.01.2020 06:31

English, 04.01.2020 06:31



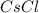 :
: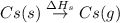
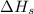 = sublimation energy of calcium
= sublimation energy of calcium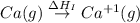
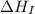 = ionization energy of calcium
= ionization energy of calcium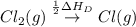
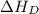 = dissociation energy of chlorine
= dissociation energy of chlorine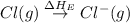
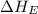 = electron affinity energy of chlorine
= electron affinity energy of chlorine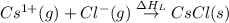
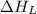 = lattice energy of calcium chloride
= lattice energy of calcium chloride