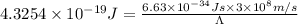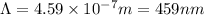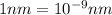Physics, 20.07.2019 03:20 HoodieHeem
The energy difference between the 5d and the 6s sublevels in gold accounts for its color. if this energy difference is about 2.7 ev (electron volt; 1 ev = 1.602 × 10−19 j), calculate the wavelength of light absorbed in the transition of an electron from the 5d subshell to the 6s subshell. round the answer to the correct number of significant figures.

Answers: 2


Another question on Physics

Physics, 22.06.2019 12:30
What is the power rating of the lightbulb if 3.0 a flow through it when connected to a 15 v battery
Answers: 1

Physics, 22.06.2019 15:30
How many neutrons does element x if it’s atomic number is 28 and its mass number is 87
Answers: 1

Physics, 22.06.2019 16:30
Humidity is to blame for that muggy, steamy feeling you experience on some hot summer days. what gas in the atmosphere causes humidity? a) oxygen b) hydrogen c) nitrogen d) water vapor
Answers: 1

Physics, 22.06.2019 21:00
Ascientist wants to test ways to reduce pollution in lake . what is the bestway of doing this
Answers: 2
You know the right answer?
The energy difference between the 5d and the 6s sublevels in gold accounts for its color. if this en...
Questions



Mathematics, 26.07.2019 20:00

English, 26.07.2019 20:00

Mathematics, 26.07.2019 20:00


Mathematics, 26.07.2019 20:00

Spanish, 26.07.2019 20:00




History, 26.07.2019 20:00



History, 26.07.2019 20:00

Mathematics, 26.07.2019 20:00


Mathematics, 26.07.2019 20:00


Mathematics, 26.07.2019 20:00

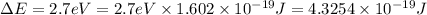
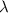
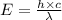
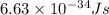
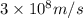
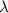 = wavelength of the photon
= wavelength of the photon