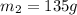Answers: 2


Another question on Physics

Physics, 21.06.2019 15:30
Taylor's experiment: 1. accurately measured the speed of light 2. locked together the phases of two light sources so that the interference pattern would not shift 3. showed that man's reaction time was much slower than the speed of light 4. showed that individual photons have wave characteristics
Answers: 2

Physics, 22.06.2019 03:10
Athin plate moves between two parallel, horizontal, stationary flat surfaces at a constant velocity of v = 7.5 m/s. the two stationary surfaces are spaced 4 cm apart, and the medium between them is filled with oil whose viscosity is 0.9 n·s/m2. the part of the plate immersed in oil at any given time is 2 m long and 0.5 m wide. if the plate moves through the mid-plane between the surfaces, determine the force required to maintain this motion. what would your response be if the plate was 1 cm from the bottom surface (h2) and 3 cm from the top surface (h1)? if the plate moves through the mid-plane between the surfaces, the force required to maintain the motion will be n. if the plate was 1 cm from the bottom surface (h2) and 3 cm from the top (h1) surface, the force required to maintain the motion would be n.
Answers: 2

Physics, 22.06.2019 05:30
What do you think car designers do if the damage caused by a crash test is too severe?
Answers: 1

Physics, 22.06.2019 09:30
How would a small bar magnet be oriented when placed at position x?
Answers: 2
You know the right answer?
A100.0 g ice cube at -10 degrees celsius is placed in an aluminum cup whose initial temperature is 7...
Questions


Mathematics, 25.09.2021 03:50


Spanish, 25.09.2021 03:50

Mathematics, 25.09.2021 03:50


Mathematics, 25.09.2021 03:50

Mathematics, 25.09.2021 03:50



English, 25.09.2021 03:50

Physics, 25.09.2021 03:50


Mathematics, 25.09.2021 03:50

Mathematics, 25.09.2021 03:50

Health, 25.09.2021 03:50


English, 25.09.2021 03:50

Mathematics, 25.09.2021 03:50

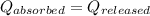
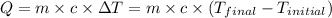
![m_1\times c\times (T_{final}-T_1)=-[m_2\times c\times (T_{final}-T_2)]](/tpl/images/0082/1939/b0e58.png)
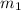 = mass of ice = 100 g
= mass of ice = 100 g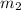 = mass of aluminium cup =? g
= mass of aluminium cup =? g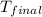 = final temperature =
= final temperature =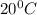
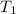 = temperature of ice =
= temperature of ice = 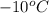
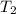 = temperature of aluminium cup=
= temperature of aluminium cup= 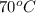
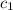 = specific heat of ice=
= specific heat of ice= 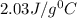
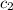 = specific heat of aluminium cup =
= specific heat of aluminium cup = 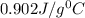
![[100\times 2.03\times (20-(-10))]=-[m_2\times 0.902\times (20-70)]](/tpl/images/0082/1939/c1f76.png)