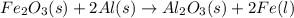Physics, 05.02.2020 01:47 snikergrace
Iron oxide reacts with aluminum to give aluminum oxide and iron. what kind of chemical reaction is this? a. combustion b. decomposition c. replacement d. synthesis

Answers: 1


Another question on Physics

Physics, 22.06.2019 00:00
Explain to someone who knows nothing about fossils the study of invertebrate paleontology and how it scientists understand the history of the earth. be sure your explanation contains a minimum of two complete sentences.
Answers: 2

Physics, 22.06.2019 00:10
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3

Physics, 22.06.2019 04:40
Which statement best describes copper? a. it has a low resistance and prevents charges from moving freely. b it has a high resistance and allows charges to move freely. c. it has a high resistance and prevents charges from moving freely. d. it has a low resistance and allows charges to move freely
Answers: 1

Physics, 22.06.2019 04:50
The position of a crate sliding down a ramp is given by x=(0.05t^3) m, y=(1.7t^2) m, z=(6−0.85t^5/2) m, where t is in seconds. (a) determine the magnitude of the crate's velocity when t = 2 s. (b) determine the magnitude of the crate's acceleration when t = 2 s.
Answers: 2
You know the right answer?
Iron oxide reacts with aluminum to give aluminum oxide and iron. what kind of chemical reaction is t...
Questions





Mathematics, 15.12.2021 21:20

English, 15.12.2021 21:20

History, 15.12.2021 21:20


Mathematics, 15.12.2021 21:20


Spanish, 15.12.2021 21:20



Mathematics, 15.12.2021 21:20





Mathematics, 15.12.2021 21:20