
Physics, 05.07.2019 03:20 0Brittany0
Suppose 8.50 ✕ 10^5 j of energy are transferred to 1.63 kg of ice at 0°c. the latent heat of fusion and specific heat of water are lf = 3.33 ✕ 105 j/kg and c = 4186 j (kg · °c) . hint (a) calculate the energy (in j) required to melt all the ice into liquid water. (enter your answer to at least three significant figures.) j (b) how much energy (in j) remains to raise the temperature of the liquid water? (enter your answer to at least three significant figures.) j (c) determine the final temperature of the liquid water in celsius. °c

Answers: 3


Another question on Physics

Physics, 21.06.2019 20:30
Asmall metal bar, whose initial temperature was 10° c, is dropped into a large container of boiling water. how long will it take the bar to reach 70° c if it is known that its temperature increases 2° during the first second? (the boiling temperature for water is 100° c. round your answer to one decimal place.) sec how long will it take the bar to reach 98° c? (round your answer to one decimal place.)
Answers: 2

Physics, 22.06.2019 02:30
The human body stores chemical energy from digested food. some of the chemical energy is converted into mechanical energy when the body moves. which statement best supported by this information
Answers: 3


Physics, 22.06.2019 16:00
Which composition of water moves to begin a deepwater current?
Answers: 2
You know the right answer?
Suppose 8.50 ✕ 10^5 j of energy are transferred to 1.63 kg of ice at 0°c. the latent heat of fusion...
Questions










Health, 29.08.2019 12:30


Chemistry, 29.08.2019 12:30


History, 29.08.2019 12:30


History, 29.08.2019 12:30



Mathematics, 29.08.2019 12:30

Mathematics, 29.08.2019 12:30

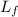 = Latent heat of fusion of ice to water = 3.33 x 10⁵ J/kg
= Latent heat of fusion of ice to water = 3.33 x 10⁵ J/kg 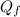 = Energy required to melt the ice
= Energy required to melt the ice 

