
Physics, 03.07.2019 23:30 itaheart101
A40 g block of ice is cooled to -69°c and is then added to 590 g of water in an 80 g copper calorimeter at a temperature of 22°c. determine the final temperature of the system consisting of the ice, water, and calorimeter. remember that the ice must first warm to 0°c, melt, and then continue warming as water. the specific heat of ice is 0.500 cal/g·°c = 2090 j/kg°c.

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:20
According to newton's second law, when the same force is applied to two objects of different masses, a the object with greater mass will experience a great acceleration, and the object with less mass will experience an even greater acceleration, b. the object with greater mass will experience a smaller acceleration, and the object with less mass will experience a greater acceleration, c. the object with greater mass will experience a greater acceleration, and the object with less mass will experience a smaller acceleration, d. the object with greater mass will experience a small acceleration, and the object with less mass will experience an even smaller acceleration.
Answers: 1

Physics, 22.06.2019 06:00
A1,700kg car is being used to give a 1,400kg car a push start by exerting a force of 140n the impulse on the smaller car during the 30.0s of contact is +670kg*m/s. what is the impulse of the smaller car on the larger car? -814 kg*m/s 0kg *m/s -670kg*m/s -550kg*m/s
Answers: 1

Physics, 22.06.2019 17:40
Which describes an object in projectile motion? check all that apply.a.gravity acts to pull the object downb.the object moves in a straight path.c.the forward velocity of the object is 0 m/s.d.the object’s inertia carries it forward.e.the path of the object is curved.
Answers: 2

Physics, 22.06.2019 22:30
Compare the patterns of the two waves shown in the image. which statement is true about these waves?
Answers: 1
You know the right answer?
A40 g block of ice is cooled to -69°c and is then added to 590 g of water in an 80 g copper calorime...
Questions




Mathematics, 04.04.2020 12:29

Medicine, 04.04.2020 12:29




Mathematics, 04.04.2020 12:29


Geography, 04.04.2020 12:29







Biology, 04.04.2020 12:31

Computers and Technology, 04.04.2020 12:31

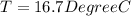
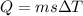
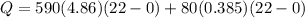
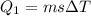
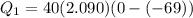
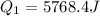
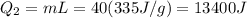
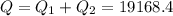
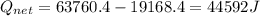
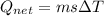
![44592 = (590 + 40)(4.186)(T - 0) + 80(0.385)(T - 0)[tex]T = 16.7 Degree C](/tpl/images/0048/1109/c6b32.png)


