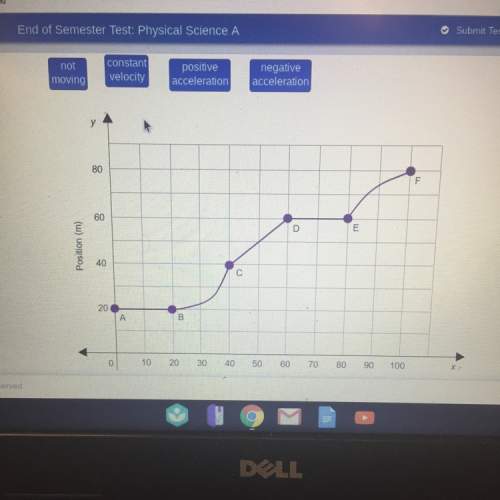
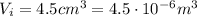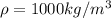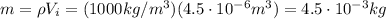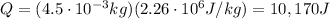Physics, 15.01.2020 05:31 gabbystar517
4.5 cm3 of water is boiled at atmospheric pressure to become 4048.3 cm3 of steam, also at atmospheric pressure. calculate the work done by the gas during this process. the latent heat of vaporization of water is 2.26 × 106 j/kg . answer in units of j. 006 (part 2 of 3) 10.0 points find the amount of heat added to the water to accomplish this process. answer in units of j.

Answers: 1


Another question on Physics

Physics, 22.06.2019 08:30
Determine the age of a fossil if it had only one eighth of its original carbon-14 content remaining?
Answers: 3

Physics, 22.06.2019 09:00
An open cart is moving along a straight frictionless horizontal track. when rain starts falling vertically into the cart, what happens to the speed of the cart?
Answers: 3

Physics, 22.06.2019 10:30
An insulated 40 ft^3 rigid tank contains air at 50 psia and 120°f. a valve connected to the tank is now opened, and air is allowed to escape until the pressure inside drops to 25 psia. the air temperature during this process is kept constant by an electric resistance heater placed in the tank. determine the electrical work done during this process.
Answers: 2

Physics, 22.06.2019 11:50
Select all that applywhat are some basic resources a family is expected to provide for children? educationclothesspending
Answers: 2
You know the right answer?
4.5 cm3 of water is boiled at atmospheric pressure to become 4048.3 cm3 of steam, also at atmospheri...
Questions

Chemistry, 12.04.2021 20:40



Mathematics, 12.04.2021 20:40

History, 12.04.2021 20:40




Mathematics, 12.04.2021 20:40

Mathematics, 12.04.2021 20:40

Computers and Technology, 12.04.2021 20:40


Mathematics, 12.04.2021 20:40




Mathematics, 12.04.2021 20:40


Physics, 12.04.2021 20:40

Mathematics, 12.04.2021 20:40

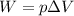
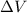 is the change in volume of the gas
is the change in volume of the gas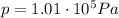 (atmospheric pressure)
(atmospheric pressure)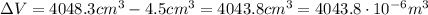 is the change in volume
is the change in volume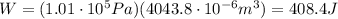
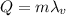
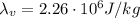 is the specific latent heat of vaporization
is the specific latent heat of vaporization