
Physics, 23.01.2020 02:31 Lianabel0517
25 points (usatestprep)
two iron balls of different mass are heated to 100°c and dropped in water. if the same amount of heat is lost by the two balls to water, what can be said about the final temperatures of the two balls? (heat lost = mcpδt, where m = mass of the object, cp = specific heat capacity of the material, and δt = change in temperature).
a) both balls will have the same temperature as the heat lost by both balls is the same.
b) both balls will have the same temperature as the specific heat capacities of both balls are the same.
c) the lighter ball will have a higher temperature because the change of temperature is directly proportional to mass.
d) the heavier ball will have a higher temperature because the change of temperature is inversely proportional to mass.

Answers: 3


Another question on Physics

Physics, 21.06.2019 22:10
How would doubling the made of an object change the objects potential energy
Answers: 2

Physics, 22.06.2019 04:40
Steam enters an adiabatic diffuser at 150 kpa and 1208c with a velocity of 550 m/s. determine the minimum velocity that the steam can have at the outlet when the outlet pressure is 300 kpa.
Answers: 3

Physics, 22.06.2019 22:00
Agirl throws a ball. if the ball's acceleration is 12 m/sec/sec and its mass is 0.5 kg, how much force did the girl apply to the ball?
Answers: 1

Physics, 23.06.2019 02:00
Explain the difference in reactivity of the elements based on their electron configurations.
Answers: 1
You know the right answer?
25 points (usatestprep)
two iron balls of different mass are heated to 100°c and dropp...
two iron balls of different mass are heated to 100°c and dropp...
Questions



History, 23.05.2020 21:01

Mathematics, 23.05.2020 21:01


History, 23.05.2020 21:01






Mathematics, 23.05.2020 21:01



Mathematics, 23.05.2020 21:01


Mathematics, 23.05.2020 21:01


Mathematics, 23.05.2020 21:01

History, 23.05.2020 21:01

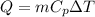
 is the change in temperature of each ball
is the change in temperature of each ball must be the same for the two balls. So, the mass and the change in temperature are inversely proportional: therefore, the heavier ball will have a smaller change in temperature. And since both balls starts from the same temperature, 100 C, this means that the heavier ball will reach a higher temperature than the lighter ball.
must be the same for the two balls. So, the mass and the change in temperature are inversely proportional: therefore, the heavier ball will have a smaller change in temperature. And since both balls starts from the same temperature, 100 C, this means that the heavier ball will reach a higher temperature than the lighter ball.

