
Physics, 18.10.2019 14:30 dianepowers1
The lowest pressure attainable using the best available vacuum techniques is about 10^−12 n/m^2.
at such a pressure, how many molecules are there per cm^3 at 19 °c?

Answers: 1


Another question on Physics

Physics, 22.06.2019 05:10
What do elements in a family tend to share. a.) similar periods b.) similar groups c.) similar atomic symbols d.) similar chemical properties and characteristics
Answers: 2

Physics, 22.06.2019 12:30
Consider a 1000 w iron whose base plate is made of 0.5 cm thick aluminum alloy 2024-t6 (ρ = 2770 kg/m3 and cp = 875 j/kg°c). the base plate has a surface area of 0.03 m2. initially, the iron is in thermal equilibrium with the ambient air at 22°c. assuming 90% of the heat generated in the resistance wires is transferred to the plate, determine the minimum time needed for the plate temperature to reach 200°c.
Answers: 1

Physics, 22.06.2019 15:50
If the work required to stretch a spring 3 ft beyond its natural length is 15 ft-lb, how much work is needed to stretch it 27 in. beyond its natural length?
Answers: 1

Physics, 22.06.2019 22:00
Aparticle of mass m is placed in a one-dimensional box of length l. the box is so small that the particle’s motion is relativistic, so that e p2/2m is not valid. (a) derive an expression for the energy levels of the particle using the relativistic energy–momentum relation and the quantization of momentum that derives from connement. (b) if the particle is an electron in a box of length l 1.00 1012 m, nd its lowest possible kinetic energy. by what percent is the nonrelativistic formula for the energy in error?
Answers: 1
You know the right answer?
The lowest pressure attainable using the best available vacuum techniques is about 10^−12 n/m^2.
Questions


Mathematics, 06.11.2020 20:50

History, 06.11.2020 20:50


Mathematics, 06.11.2020 20:50

Mathematics, 06.11.2020 20:50

Health, 06.11.2020 20:50

Mathematics, 06.11.2020 20:50



Mathematics, 06.11.2020 20:50



Biology, 06.11.2020 20:50

Physics, 06.11.2020 20:50

English, 06.11.2020 20:50

English, 06.11.2020 20:50


Spanish, 06.11.2020 20:50

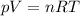
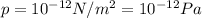 is the lowest pressure attainable
is the lowest pressure attainable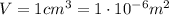 is the volume we are considering
is the volume we are considering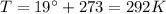 is the absolute temperature
is the absolute temperature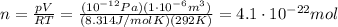
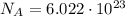 (avogadro number)
(avogadro number)


