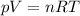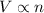Physics, 27.09.2019 12:00 Ryantimes2
Which of the following laws concerning gases is misstated? a. in a mixture of gases, the total pressure of the gases equals the sum of the individual pressures of each gas. b. at fixed temperature and pressure, the volume of a gas is inversely proportional to the number of moles of that gas. c. at fixed pressure, the volume of a gas is directly proportional to its temperature in kelvin. d. at fixed temperature, the volume of a gas is inversely proportional to its pressure. e. the average translational kinetic energy of the molecules of a gas is directly proportional to the temperature in kelvin.

Answers: 1


Another question on Physics

Physics, 22.06.2019 03:00
Which of the following harmful chemicals are found in tobacco smoke? a. carbon monoxide b. carbon dioxide c. nicotine b. carbon dioxide d. both a and c
Answers: 2

Physics, 22.06.2019 08:00
A0.580-kg rock is tied to the end of a string and is swung in a circle with a radius of 0.500 meters. the velocity of the rock is 4.50 m/s. what is the centripetal force acting on the rock? 15.5 n 5.22 n 69.8 n 23.5 n
Answers: 2

Physics, 22.06.2019 10:10
Apair of 10μf capacitors in a high-power laser are charged to 1.7 kv.a. what charge is stored in each capacitor? b. how much energy is stored in each capacitor?
Answers: 2

Physics, 23.06.2019 00:00
What word chemical equation describes cavendish’s experiment with zinc?
Answers: 1
You know the right answer?
Which of the following laws concerning gases is misstated? a. in a mixture of gases, the total pres...
Questions

Chemistry, 26.07.2021 09:00

Chemistry, 26.07.2021 09:00





Mathematics, 26.07.2021 09:00

Mathematics, 26.07.2021 09:00


Business, 26.07.2021 09:00

Mathematics, 26.07.2021 09:00


Mathematics, 26.07.2021 09:10

Mathematics, 26.07.2021 09:10

Mathematics, 26.07.2021 09:10



Business, 26.07.2021 09:10


English, 26.07.2021 09:10