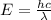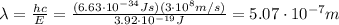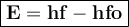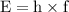Physics, 26.10.2019 16:43 acavalieri72
Image that the radiation emitted by the nitrogen at a frequency of 8.88×1014 hz is absorbed by an electron in a molecule of methyl salicylate. as a result, the electron in the wintergreen oil molecule jumps to an excited state. before returning to its ground state, the electron drops to an intermediate energy level, releasing two-thirds of the energy previously absorbed and emitting a photon. what is the wavelength of the photon emitted by the wintergreen oil molecule?

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:30
An object starts from rest at point f and speeds up continuously as it moves around an oval. a. choose a point about 1/8 th of the way around the oval from point f, and label it point g. draw a vector to represent the velocity of the object at point g. b. determine the change in velocity vector between points f and g.
Answers: 1

Physics, 22.06.2019 04:10
An initially unpolarized light if intensity 500 w/m^2 passes through four polarizers. the transmission axis between adjacent polarizers is 45∘. what percentage of the initial intensity is transmitted by the system?
Answers: 2

Physics, 22.06.2019 09:00
In the first law of thermodynamics, triangle e=q-w, what does q stand for
Answers: 1

Physics, 22.06.2019 09:30
True or false graphs must include scales that increase by the same amount
Answers: 3
You know the right answer?
Image that the radiation emitted by the nitrogen at a frequency of 8.88×1014 hz is absorbed by an el...
Questions

History, 03.03.2020 18:02




English, 03.03.2020 18:03

Mathematics, 03.03.2020 18:03

Mathematics, 03.03.2020 18:03



Mathematics, 03.03.2020 18:03


Physics, 03.03.2020 18:03






English, 03.03.2020 18:04

Mathematics, 03.03.2020 18:04

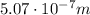 (507 nm)
(507 nm)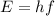
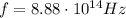 is the frequency of the photon
is the frequency of the photon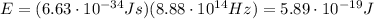
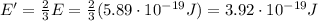
 is
is