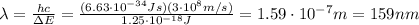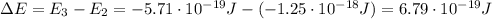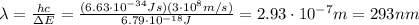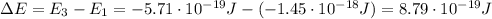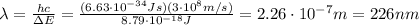Physics, 20.09.2019 10:30 JuanTorres7
Be sure to answer all parts. consider the following energy levels of a hypothetical atom: e4 −2.01 × 10−19 j e3 −5.71 × 10−19 j e2 −1.25 × 10−18 j e1 −1.45 × 10−18 j (a) what is the wavelength of the photon needed to excite an electron from e1 to e4? × 10 m (b) what is the energy (in joules) a photon must have in order to excite an electron from e2 to e3? × 10 j (c) when an electron drops from the e3 level to the e1 level, the atom is said to undergo emission. calculate the wavelength of the photon emitted in this process. × 10 m

Answers: 1


Another question on Physics

Physics, 22.06.2019 02:30
Which type of bond is found between the atoms of a molecule? a. metallic b. delocalized electron c. covalent d. ionic
Answers: 1

Physics, 22.06.2019 17:30
Atruck driver is attempting to deliver some furniture. first, he travels 8 km east, and then he turns around and travels 3 km west. finally, he turns again and travels 13 km to his destination. what is the drivers distance
Answers: 1

Physics, 22.06.2019 19:40
Currents in dc transmission lines can be 100 a or higher. some people are concerned that the electromagnetic fields from such lines near their homes could pose health dangers. for a line that has current 150 a and a height of 8.0 m above the ground, what magnetic field does the line produce at ground level? express your answer in teslas and as a percentage of the earth’s magnetic field, which is 0.50 g. is this value cause for worry?
Answers: 1

You know the right answer?
Be sure to answer all parts. consider the following energy levels of a hypothetical atom: e4 −2.01...
Questions


Mathematics, 20.05.2021 01:00


Mathematics, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Biology, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

History, 20.05.2021 01:00


Mathematics, 20.05.2021 01:00

Biology, 20.05.2021 01:00




Mathematics, 20.05.2021 01:00

Social Studies, 20.05.2021 01:00

English, 20.05.2021 01:00


Chemistry, 20.05.2021 01:00

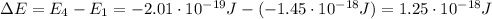
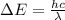
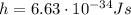 is the Planck constant
is the Planck constant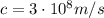 is the speed of light
is the speed of light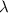 is the wavelength of the photon
is the wavelength of the photon