
A. compare solids, liquids, and gases in terms of the way their volumes change (or don't change) when placed into different containers.
b. a sample of gas in a rigid container that cannot change volume. use the kinetic molecular theory to explain why the pressure in the container will decreases as the temperature rises.
c. write one or two sentences to describe how heat will flow between two objects of different temperatures that are touching.
d. write one or two sentences to compare the internal energy of a molecular gas with the internal energy of a monatomic gas.
e. potential energy from intermolecular forces is included in the internal energies of which states of matter?
f. what is the average velocity of atoms in 1.00 mol of krypton (a monatomic gas) at 347 k? for m, use 0.0838
g. what is the internal energy of 4.00 mol of diatomic nitrogen gas n2, at 455 k?

Answers: 1


Another question on Physics

Physics, 22.06.2019 10:00
The frequencies refer to the sample data collected from a population of interest when performing a hypothesis test comparing two or more population proportions.
Answers: 2

Physics, 22.06.2019 14:40
What is the orbital period of a spacecraft in a low orbit near the surface of mars? the radius of mars is 3.4×106m.
Answers: 2


Physics, 22.06.2019 16:00
Suppose a soccer ball is kicked from the ground at an angle 20.0º above the horizontal at 8.00 m/s. the y-velocity is determined to be 2.74 m/s. how long will the ball be in the air? assume the ball lands at the same height at which it was kicked.
Answers: 2
You know the right answer?
A. compare solids, liquids, and gases in terms of the way their volumes change (or don't change) whe...
Questions




Mathematics, 17.02.2020 16:15


Mathematics, 17.02.2020 16:16


Business, 17.02.2020 16:17

Mathematics, 17.02.2020 16:18

Chemistry, 17.02.2020 16:20









Chemistry, 17.02.2020 16:26

Mathematics, 17.02.2020 16:26

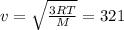 m/s
m/s

