
Physics, 29.06.2019 04:00 Christsflower601
What is the trend in sizes of the ions n3−, o 2−, f− and why does the trend exist? 1. n 3− > o 2− > f −; the effective nuclear charge is smallest at n3− and the largest at f −. 2. n 3− > o 2− > f −; for an isoelelectronic series, the ionic size decreases as the p + e− ratio decreases. 3. impossible to tell, since ionic radii for different elements cannot be compared; ions can only be compared to the neutral atom. 4. f − > o 2− > n 3−; in an isoelelectronic se-?

Answers: 1


Another question on Physics


Physics, 22.06.2019 10:30
The freezing and boiling point of a substance changes as the air pressure around it changes. for example, at a lower air pressure (higher altitude) it is easier for water molecules to escape from liquid into the air. in a high altitude city such as denver, colorado compared to a sea-level city such as houston, texas, water
Answers: 2


Physics, 22.06.2019 16:40
Which are true about beta reactions? there will be more than one answer. a. it has no change b. it doesn't change the mass of the number c. it has a mass of 0.0005 atoms d. it causes transmutation.
Answers: 3
You know the right answer?
What is the trend in sizes of the ions n3−, o 2−, f− and why does the trend exist? 1. n 3− > o...
Questions







Mathematics, 26.02.2020 18:59





Computers and Technology, 26.02.2020 18:59


English, 26.02.2020 18:59


English, 26.02.2020 19:00




Computers and Technology, 26.02.2020 19:00

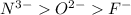 ; the effective nuclear charge is smallest at
; the effective nuclear charge is smallest at 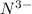 and the largest at
and the largest at 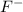
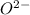 : Atomic number or number of electrons or number of protons in Oxygen is 8. As
: Atomic number or number of electrons or number of protons in Oxygen is 8. As 

