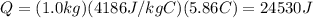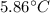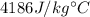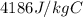Physics, 30.06.2019 03:00 greenbyron88
The mass of the water to 1.0 kg (1000 g). the cylinder has a mass of 5.0 kg and a height of 500 m. the temperature changed from 25 degrees celsius to 30.86 degrees celsius in the experiment. specific heat can be calculated using the following equation: q=mc(delta t) what is the gravitational potential energy of the cylinder? if no energy is lost, how much heat energy is added to the water? what is the mass of the water? what is the temperature change of the water? what is the specific heat of the water?

Answers: 1


Another question on Physics

Physics, 22.06.2019 10:00
Because air contracts as it cools, the air pressure inside a freezer is typically lower than on the outside. why do ice cubes inside a freezer tend to shrink over time? a. the ice dissolves oxygen from the air, forming a denser crystalline matrix.b. the ice reacts chemically with carbon dioxide in the air, forming gaseous carbon compounds.c. the ice melts, and then the liquid freezes as ice crystals on the bottom of the freezer.d. the ice sublimes, and then the water vapor deposits as ice crystals on the sides of the freezer.
Answers: 1


Physics, 22.06.2019 21:30
The diagram shows a boulder rolling down a hill into a valley and then up the opposite hill. at which position does the boulder have the greatest kinetic energy? a b c d
Answers: 2

Physics, 22.06.2019 23:30
Which statement correctly describes the interaction between magnetic poles? north and south poles attract each other. north and south poles repel each other. two north poles will attract each other. two south poles will attract each other.
Answers: 1
You know the right answer?
The mass of the water to 1.0 kg (1000 g). the cylinder has a mass of 5.0 kg and a height of 500 m. t...
Questions





Mathematics, 14.07.2019 16:20










Mathematics, 14.07.2019 16:20





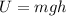
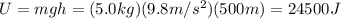
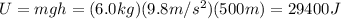
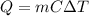
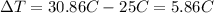 is the increase in temperature
is the increase in temperature