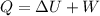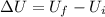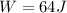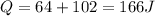Physics, 03.07.2019 19:00 dragon2565
Jin knows that the initial internal energy of a closed system is 78 j and the final internal energy is 180 j. he also knows that 64 j of energy are used to do work. to find the heat added to the system, jin completes the steps below. 1. add the initial internal energy plus the final internal energy to find the change in internal energy. 2. add the change in internal energy to the energy used to do work. 3. write the answer in joules. which best describes jin's error? a )for step 1, he should have used 180 j as the change in internal energy. b )for step 1, he should have subtracted 78 j from 180 j to find the change in internal energy. c )for step 2, he should have subtracted the change in internal energy from the energy used to do work. d )for step 2, he should have subtracted the energy used to do work from the change in internal energy.

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:30
Asample of a gaseous substance changes into liquid. this process is called (blank) and it takes place when heat is (blank) a gaseous system
Answers: 2

Physics, 21.06.2019 20:30
Which of the following is a source of heat for magma formation? magma plumes in the continental crust friction due to divergence friction due to subduction magma plumes in the oceanic crust
Answers: 1


Physics, 22.06.2019 08:30
What object a collides with object b and bounces back its final momentum is?
Answers: 1
You know the right answer?
Jin knows that the initial internal energy of a closed system is 78 j and the final internal energy...
Questions


Mathematics, 24.01.2020 10:31




History, 24.01.2020 10:31

Mathematics, 24.01.2020 10:31



English, 24.01.2020 10:31


Mathematics, 24.01.2020 10:31

History, 24.01.2020 10:31



Biology, 24.01.2020 10:31



French, 24.01.2020 10:31

Mathematics, 24.01.2020 10:31