Answer = brainliest
pleaassee
...

Answers: 1


Another question on Physics

Physics, 22.06.2019 00:10
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3

Physics, 22.06.2019 05:10
A- a of 8.00 cm a of 34.0 nc. a , -, a of 25.0 nc is on of a of 15.0 cm .be toto aof ? a) b) c) j d) e)
Answers: 1

Physics, 22.06.2019 13:20
It is reasonable to assume that the bulk modulus of blood is about the same as that of water (2.2 gpa). as one goes deeper and deeper in the ocean, the pressure increases by 10000 pa for every meter below the surface. if a diver goes down 80.0 m in the ocean, by how much does each cubic centimeter of her blood change in volume? give the answer in cubic centimeters (actually one cubic centimeter equals one milliliter).
Answers: 2

Physics, 22.06.2019 14:40
According to valence bond theory, which orbitals overlap in the formation of the bond in hf according to valence bond theory, which orbitals overlap in the formation of the bond in hf 2s on h and 2p on f 1s on h and 2s on f 1s on h and 1p on f 1s on h and 2p on f 1s on h and 3p on f
Answers: 3
You know the right answer?
Questions







World Languages, 22.10.2020 02:01



Mathematics, 22.10.2020 02:01


Mathematics, 22.10.2020 02:01

Computers and Technology, 22.10.2020 02:01



Computers and Technology, 22.10.2020 02:01


Computers and Technology, 22.10.2020 02:01



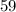 kg).
kg).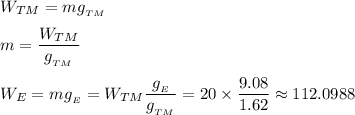
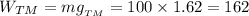
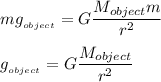
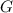 and
and  are constants,
are constants, 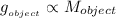 . In other words, the more massive the object is, the bigger its gravitational acceleration (obviously, the lighter the object is, the lesser its gravitational acceleration)
. In other words, the more massive the object is, the bigger its gravitational acceleration (obviously, the lighter the object is, the lesser its gravitational acceleration)

