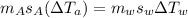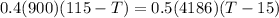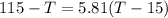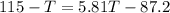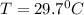Physics, 11.07.2019 00:00 brittanyjacob8
A0.4 kilogram sample of aluminum at 115 degrees celsius is put into a container containing 0.5 kilograms of water at 15 degrees celsius. neglecting the small amount of energy absorbed by the container and knowing that the specific heat of aluminum is 900 kj/kg*c, and the specific heat of the water is 4186 kj/kg*c answer the following question. compared to the heat liberated by the aluminum, the heat absorbed by the water is

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:40
In physics the desire of an object to keep doing what it is doing is termed?
Answers: 1


Physics, 22.06.2019 10:30
Air is to be preheated by hot exhaust gases in a cross-flow heat exchanger before it enters the furnace. air enters the heat exchanger at 95 kpa and 20°c at a rate of 0.6 m^3/s. the combustion gases (cp = 1.10 kj/kg°c) enter at 160°c at a rate of 0.95 kg/s and leave at 95°c. determine the rate of heat transfer to the air and its outlet temperature.
Answers: 2

Physics, 22.06.2019 14:30
Which of the following bonds would be most polar? a. c-i b. c-br c. c-cl d. c-f e. c-o
Answers: 1
You know the right answer?
A0.4 kilogram sample of aluminum at 115 degrees celsius is put into a container containing 0.5 kilog...
Questions



Mathematics, 31.03.2021 17:00



Mathematics, 31.03.2021 17:00



Mathematics, 31.03.2021 17:00

Mathematics, 31.03.2021 17:00

Mathematics, 31.03.2021 17:00

Mathematics, 31.03.2021 17:00



Mathematics, 31.03.2021 17:00

English, 31.03.2021 17:00


Mathematics, 31.03.2021 17:00

Chemistry, 31.03.2021 17:00