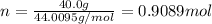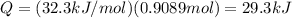Answers: 1


Another question on Physics

Physics, 22.06.2019 01:40
Crowbar of 5 metre is used to lift an object of 800 metre if the effort arm is 200cm calculate the force applied
Answers: 1

Physics, 22.06.2019 19:30
The ability to make things happen is also called a. heat b. force c. matter d. energy
Answers: 2


Physics, 23.06.2019 01:30
Relative to the ground below, how many joules of potential energy does a 1000-kg boulder have at the top of a 5-m ledge?
Answers: 1
You know the right answer?
Calculate the amount of heat required to completely sublime 40.0 g of solid dry ice (co2) at its sub...
Questions


Biology, 10.04.2020 21:53


Social Studies, 10.04.2020 21:53




Computers and Technology, 10.04.2020 21:53

Mathematics, 10.04.2020 21:53

English, 10.04.2020 21:53





Physics, 10.04.2020 21:53

Mathematics, 10.04.2020 21:53



Mathematics, 10.04.2020 21:53

World Languages, 10.04.2020 21:53