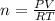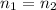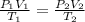Physics, 14.07.2019 16:30 keaton3410
According to avogadro's law, the volume of a gas is inversely related to the number of moles at constant temperature and pressure the volume of a gas is inversely related to the number of moles at standard temperature and pressure the volume of a gas depends only on the temperature and pressure the volume of a gas depends only on the number of moles in the sample the volume of a gas is directly related to the number of moles at constant temperature and pressure

Answers: 1


Another question on Physics

Physics, 21.06.2019 21:30
Write the equation for momentum, first using symbols for the variables, then using words for the variables.
Answers: 2

Physics, 22.06.2019 01:20
Acar moves a distance of 50.0 km west, followed by a distance of 64.9 km north what was the magnitude of the displacement of the car, in units of kilometers?
Answers: 2

Physics, 22.06.2019 01:30
John throws .4 kg ball with velocity of 18 m/s. hits .2 kg bottle and bottle flies 25 m/s. how fast is ball traveling after hitting the bottle?
Answers: 1

Physics, 22.06.2019 05:20
Alfred pushes on a heavy box, but cannot move it. the box has a lot of inertia motion friction gravity
Answers: 1
You know the right answer?
According to avogadro's law, the volume of a gas is inversely related to the number of moles at con...
Questions







Mathematics, 28.05.2021 03:00


Mathematics, 28.05.2021 03:00






Biology, 28.05.2021 03:00