Mathematics, 07.07.2021 16:00 AshlynPlayz45
(NEED THIS ASAP)
Tests show that the hydrogen ion concentration of a sample of apple juice is 0.0003 and that of ammonia is 1.3 x 10-9. Find the approximate pH of each liquid using the formula pH = -log (H+), where (H+) is the hydrogen ion concentration The pH value of the apple juice is___ The pH value of ammonia is
1.pH of apple juice
A. 8.11
B. 1.75
C. 3.5
D. 2.1
2. pH of ammonia
A. 1.1
B. 7.0
C. 5.4
D. 8.9

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 14:20
James wants to promote his band on the internet. site a offers website hosting for $4.95 per month with a $49.95 startup fee. site b offers website hosting for $9.95 per month with no startup fee. for how many months would james need to keep the website for site a to be a better choice than site b? will mark the
Answers: 1

Mathematics, 21.06.2019 19:00
What is the average diastolic blood pressure for adults
Answers: 2

Mathematics, 21.06.2019 19:30
Complete the solution of the equation. find the value of y when x equals to 6 4x+y=20
Answers: 2

Mathematics, 21.06.2019 20:30
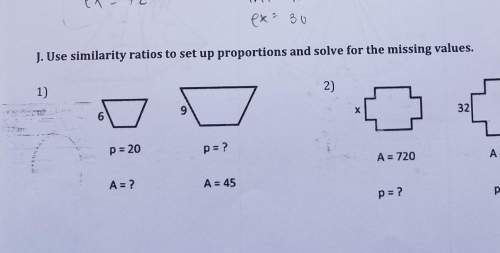
The interior angles formed by the side of a hexagon have measures of them up to 720° what is the measure of angle a
Answers: 2
You know the right answer?
(NEED THIS ASAP)
Tests show that the hydrogen ion concentration of a sample of apple juice is 0.000...
Questions


Computers and Technology, 20.07.2019 06:10







Computers and Technology, 20.07.2019 06:10




Mathematics, 20.07.2019 06:10

History, 20.07.2019 06:10




Mathematics, 20.07.2019 06:10

Biology, 20.07.2019 06:10

Mathematics, 20.07.2019 06:20