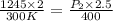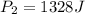Mathematics, 05.12.2019 10:31 laylah255
The pressure, p, of a gas varies directly with its temperature, t, and inversely with its volume, v, according to the equation p=nrt/v, where n is the number of molar units and r is the universal gas constant. one molar unit of gas has a pressure of about 1,245 joules at a temperature of 300 degrees kelvin and a volume of 2 liters. what is the pressure of the same number of molar units of the gas at a temperature of 400 degrees kelvin and a volume of 2.5 liters?

Answers: 3


Another question on Mathematics

Mathematics, 20.06.2019 18:02
Anew fountain in the shape of a hexagon will have 6 sides of equal length. on a scale drawing, the coordinates of the vertices of the fountain are: (7.5,5), (11.5,2), (7.5,−1), (2.5,−1), (−1.5,2), and (2.5,5). how long is each side of the fountain?
Answers: 3


Mathematics, 21.06.2019 20:00
Two line segments are shown in the figure below. suppose that the length of the line along the x-axis is 6, and the length of the hypotenuse of the triangle is 10. what is the equation of the hypotenuse line (shown in red, below)?
Answers: 3

Mathematics, 21.06.2019 21:10
Plot a point at the y-intercept of the following function on the provided graph. 3y=-5x+7 20 points
Answers: 1
You know the right answer?
The pressure, p, of a gas varies directly with its temperature, t, and inversely with its volume, v,...
Questions

Mathematics, 01.07.2021 16:40

Mathematics, 01.07.2021 16:40

Mathematics, 01.07.2021 16:40



Advanced Placement (AP), 01.07.2021 16:40

Mathematics, 01.07.2021 16:40


Business, 01.07.2021 16:40




Mathematics, 01.07.2021 16:50

Mathematics, 01.07.2021 16:50



History, 01.07.2021 16:50

Social Studies, 01.07.2021 16:50

History, 01.07.2021 16:50

Mathematics, 01.07.2021 16:50

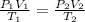 (for same value of n)
(for same value of n)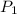 = initial pressure of gas = 1245 J
= initial pressure of gas = 1245 J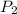 = final pressure of gas = ?
= final pressure of gas = ?
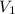 = initial volume of gas = 2 L
= initial volume of gas = 2 L
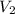 = final volume of gas = 2.5L
= final volume of gas = 2.5L
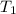 = initial temperature of gas = 300K
= initial temperature of gas = 300K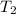 = final temperature of gas = 400K
= final temperature of gas = 400K