1. Considering the unbalanced REDOX
reaction below:
01_0,4 + Fe - Fe + c
Please balance...

Mathematics, 19.04.2021 22:30 robynlikesowls34205
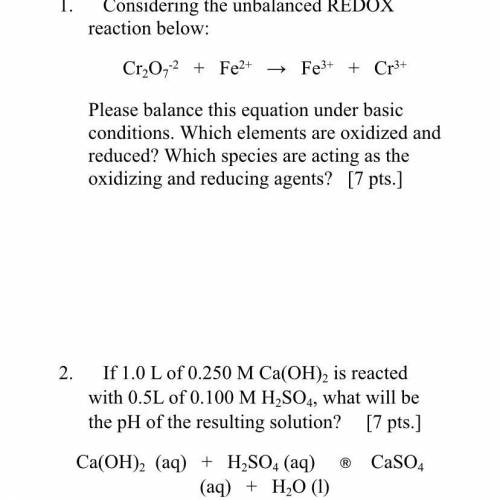
1. Considering the unbalanced REDOX
reaction below:
01_0,4 + Fe - Fe + c
Please balance this equation under basic
conditions. Which elements are oxidized and
reduced? Which species are acting as the
oxidizing and reducing agents? 17pts.]
2. If 1.0 L of 0.250 M Ca(OH), is reacted
with 0.5L of 0.100 M H SO, what will be
the pH of the resulting solution? [7pts]
Ca(OH)2 (aq) + H SO, (a) Caso
(aq) + H2O (1)

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 13:30
What is the vertical asymptotes of the function f(x)=x-2/x*2-3x-4
Answers: 1

Mathematics, 21.06.2019 16:30
Problem fathi wants to print out a pdf document that is 48 pages long. to save paper, he decides to print on both sides of each sheet and to print two pages on each side of the sheet. how many sheets of paper will he need?
Answers: 3


You know the right answer?
Questions


English, 06.12.2021 23:10

Social Studies, 06.12.2021 23:10

English, 06.12.2021 23:10




History, 06.12.2021 23:20

Mathematics, 06.12.2021 23:20



Mathematics, 06.12.2021 23:20






History, 06.12.2021 23:20




