Mathematics, 18.03.2021 02:20 emilysmith20044
A cylinder contains nitrogen gas at a temperature of 300 Kelvin and pressure of 15 atmospheres in a volume of 100 liters. Calculate the
proportionality constant k. Calculate the temperature of the nitrogen gas if a piston is
used to reduce the volume of the gas to 80 liters.
Options:
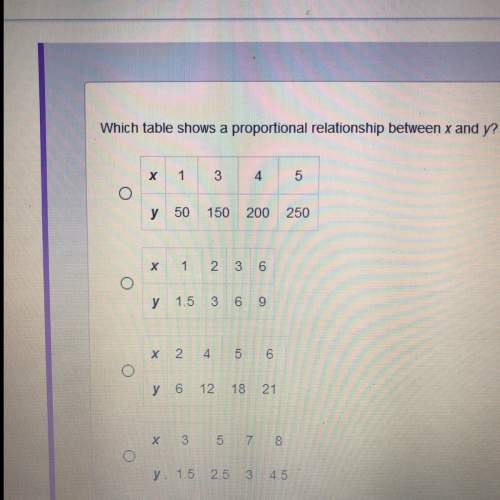
The proportionality constant is 1/3.
The temperature is 27 Kelvin when volume is 80
liters.
The proportionality constant is 3.
The temperature is 240 Kelvin when volume is 80
liters.
The proportionalitysmpnstant is 1/3.
The temperature is 240 Kelvin when volume is 80
liters.
The proportionality constant is 3.
The temperature is 27 Kelvin when volume is 80
liters.

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 15:30
Question 7 of 47 1 point what is the equation of a line with a slope of 1/2 that passes through the point, (-2,5)? o a. 1/2x+3 o b. y=1/2x+5 o c. y=x+5 o d. y=1/2x+6
Answers: 3

Mathematics, 21.06.2019 16:30
In the figure shown below, m < 40 and ab =8. which equation could be used to find x?
Answers: 2

Mathematics, 21.06.2019 19:40
Molly shared a spool of ribbon with 12 people. each person received 3 feet of ribbon. which equation can she use to find r, the number of feet of ribbon that her spool originally had?
Answers: 1

Mathematics, 21.06.2019 22:00
Here is my question! jayne is studying urban planning and finds that her town is decreasing in population by 3% each year. the population of her town is changing by a constant rate.true or false?
Answers: 2
You know the right answer?
A cylinder contains nitrogen gas at a temperature of 300 Kelvin and pressure of 15 atmospheres in a...
Questions



Mathematics, 10.01.2020 20:31




Biology, 10.01.2020 21:31

Mathematics, 10.01.2020 21:31

History, 10.01.2020 21:31


Biology, 10.01.2020 21:31

History, 10.01.2020 21:31