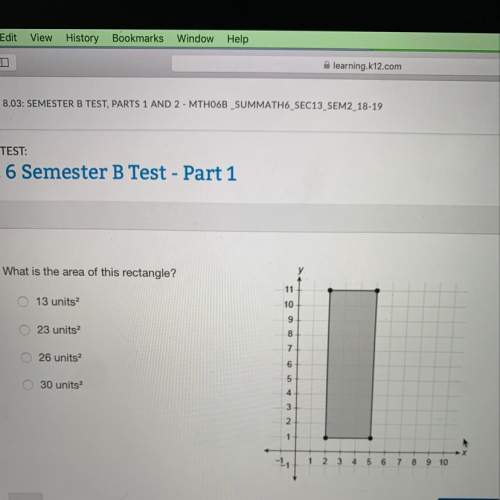
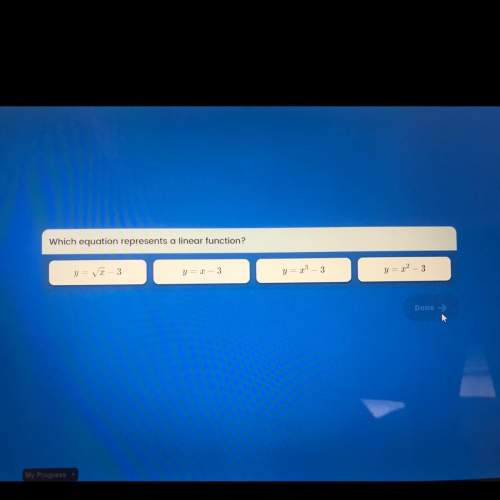
Mathematics, 01.03.2021 02:30 Izzyfizzy
A 13.57 g sample of a compound contains 4.33 g of potassium, K, 3.93 g of chlorine, Cl, and oxygen, O. Calculate the empirical formula.

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 13:00
225/100 of last years cost. write each fraction as a decimal and as a percent
Answers: 1

Mathematics, 21.06.2019 20:00
Last one and the bottom answer choice is y=(x-4)^2+1 you guys!
Answers: 1

Mathematics, 22.06.2019 01:30
Jon’s weight loss for each week of the month is 5 lbs., 2.5 lbs., and 2.5 lbs. he gained 3.5 lbs. the last week. if jon originally weighed 198 lbs., how much does he weigh now?
Answers: 1

Mathematics, 22.06.2019 04:00
Which is an equivalent equation solved for r? the circumference of a circle can be found using the formula c = 2πr. r=cπ r=c(2π) r= c over 2π r= 2π over c
Answers: 1
You know the right answer?
A 13.57 g sample of a compound contains 4.33 g of potassium, K, 3.93 g of chlorine, Cl, and oxygen,...
Questions




Chemistry, 28.12.2019 14:31

Mathematics, 28.12.2019 14:31

History, 28.12.2019 14:31




Computers and Technology, 28.12.2019 14:31

Mathematics, 28.12.2019 14:31

Arts, 28.12.2019 14:31


Geography, 28.12.2019 14:31


Mathematics, 28.12.2019 14:31

Mathematics, 28.12.2019 14:31