Concentration of NO2 = 11.95

Mathematics, 08.01.2021 01:00 krystalhurst97
For the current reaction, 2NO2 ↔ N2O4, we have:
Kc = ![\frac{[N_{2} O_{4}]}{[NO_{2}]^{2} }](/tpl/images/2047/8468/2ef86.png)
Concentration of NO2 = 11.95
Concebtration of N2O4 = 6.05
Based on the current concentrations of NO2 and N2O4, what is Kc?
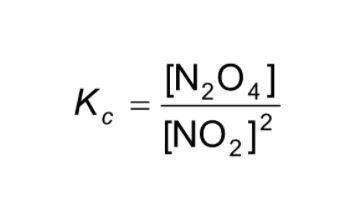

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 19:00
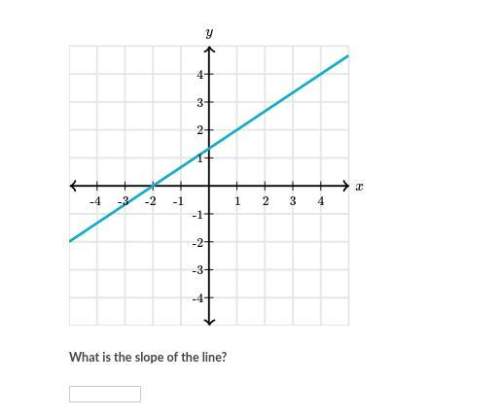
The figures below are made out of circles, semicircles, quarter circles, and a square. find the area and the perimeter of each figure and give your answers as a completely simplified exact value in terms of π (no approximations).
Answers: 1

Mathematics, 21.06.2019 20:50
There are three bags: a (contains 2 white and 4 red balls), b (8 white, 4 red) and c (1 white 3 red). you select one ball at random from each bag, observe that exactly two are white, but forget which ball came from which bag. what is the probability that you selected a white ball from bag a?
Answers: 1

Mathematics, 21.06.2019 22:00
Out of 50 students surveyed, 16 have a dog. based on these results, predict how many of the 280 students in the school have a dog.
Answers: 2

Mathematics, 22.06.2019 00:00
28 x 12 + 34 = ? it's for a test that if i fail on i will not proceed into the honor roll society i always dreamed of!me! worth 50 !
Answers: 1
You know the right answer?
For the current reaction, 2NO2 ↔ N2O4, we have:
Kc =
Concentration of NO2 = 11.95
Concentration of NO2 = 11.95
Questions

Chemistry, 23.08.2019 07:30



Health, 23.08.2019 07:30





Computers and Technology, 23.08.2019 07:30

Mathematics, 23.08.2019 07:30

English, 23.08.2019 07:30

Geography, 23.08.2019 07:30


Mathematics, 23.08.2019 07:30

French, 23.08.2019 07:30

English, 23.08.2019 07:30

Biology, 23.08.2019 07:30



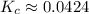
![\displaystyle K_c = \frac{[N_2O_4]}{[NO_2]^2}](/tpl/images/1020/5386/82efd.png)
![\displaystyle K_c = \frac{[6.05]}{[11.95]^2}](/tpl/images/1020/5386/714da.png) Exponents:
Exponents: ![\displaystyle K_c = \frac{[6.05]}{[142.803]}](/tpl/images/1020/5386/9a115.png) Divide:
Divide: 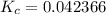 Round (Sig Figs):
Round (Sig Figs):