
Mathematics, 11.11.2020 20:20 crystaldewar55C
2C8H18 + 2502 yields 16CO2 + 18H20
In the combustion of octane, how many grams of oxygen are needed to completely react and produce 84.00 grams of
carbon dioxide? Use the periodic table to get the weights of the elements.
Drag the labels to the correct locations to complete the analysis. Each label can be used more than once.
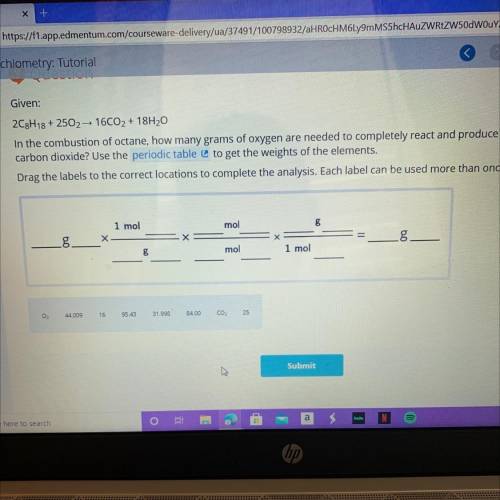

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 13:30
The graph of which of the following will be parallel to the graph of 4x – 3y = –12? a.y= 4/3x -3/2 b.6x-4y=-8 c. y=3/4x+1 d.4x- 2y=-12
Answers: 2

Mathematics, 21.06.2019 17:00
Find the measure of the interior angles of the following regular polygons: a triangle, a quadrilateral, a pentagon, an octagon, a decagon, a 30-gon, a 50-gon, and a 100-gon.
Answers: 2


Mathematics, 21.06.2019 21:30
If 1.4% of the mass of a human body is calcium, how many kilograms of calcium are there in a 165-pound man? 1.0 kg ca 5.1 kg ca 1.0 x 102 kg ca 5.1 x 102 kg ca
Answers: 1
You know the right answer?
2C8H18 + 2502 yields 16CO2 + 18H20
In the combustion of octane, how many grams of oxygen are needed...
Questions



Advanced Placement (AP), 18.02.2021 18:50


Law, 18.02.2021 18:50

History, 18.02.2021 18:50

Mathematics, 18.02.2021 18:50


Mathematics, 18.02.2021 18:50


Mathematics, 18.02.2021 18:50


Mathematics, 18.02.2021 18:50

Biology, 18.02.2021 18:50

Chemistry, 18.02.2021 18:50

History, 18.02.2021 18:50


Mathematics, 18.02.2021 18:50

Mathematics, 18.02.2021 18:50

Mathematics, 18.02.2021 18:50



