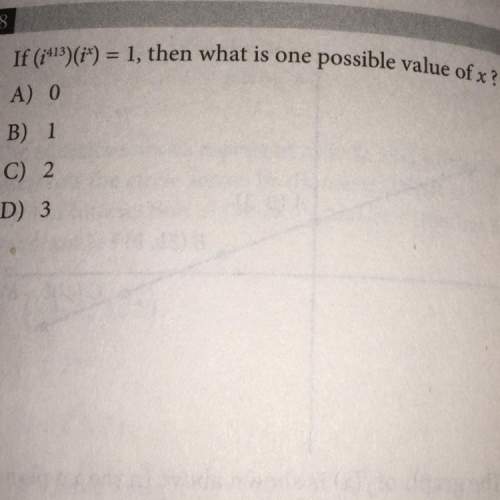Mathematics, 07.10.2020 23:01 graciemartinez9
An atom of sodium-23 (Na-23) has a net charge of
+1. Identify the number of protons, neutrons, and
electrons in the atom. Then, explain how you
determined the number of each type of particle.
Use the periodic table to help you.

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 23:00
Which rectangle if translated 6 units right and 16 units down and the rotated 90° clockwise about the point (4, -11) will result in rectangle e?
Answers: 2

Mathematics, 21.06.2019 23:30
In the diagram, ab is tangent to c, ab = 4 inches, and ad = 2 inches. find the radius of the circle.
Answers: 2

Mathematics, 22.06.2019 01:00
Mr. t has been dating his girlfriend for one year nine months and six days how many hours has mr. t been in a relationship
Answers: 1

Mathematics, 22.06.2019 01:10
Of jk j(–25, 10) k(5, –20). is y- of l, jk a 7: 3 ? a. –16 b.–11 c. –4 d.–1
Answers: 1
You know the right answer?
An atom of sodium-23 (Na-23) has a net charge of
+1. Identify the number of protons, neutrons, and<...
Questions

English, 24.06.2019 15:50

History, 24.06.2019 15:50






Mathematics, 24.06.2019 15:50


Mathematics, 24.06.2019 15:50





History, 24.06.2019 15:50





Computers and Technology, 24.06.2019 15:50