Mathematics, 08.07.2020 05:01 bella7179
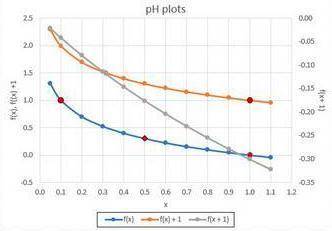
Have you ever been swimming in a pool, and your eyes were red when you get out? This means the chemicals in the pool were not adjusted correctly! More specifically, the measurement of the pool's pH was not quite right. pH is a measurement of hydronium ions and can be modeled by the function f(x) = −log10x. The variable x represents the amount of hydronium ions, and f(x) gives the pH level. Different liquids have ideal pH values that are different. Water at 25 degrees Celsius has a pH of 7. Anything that has a pH less than 7 is called acidic, and a pH above 7 is basic, or alkaline. Seawater has a pH just more than 8, whereas lemonade has a pH of approximately 3. 1. Create a graph of the pH function either by hand or using technology. Locate on your graph where the pH value is 0 and where it is 1. You may need to zoom in on your graph. 2. Use your graph to find the ideal pH level if the amount of hydronium ions is raised to 0.50. 3. The pool company developed new chemicals that transform the pH scale. Using the pH function f(x) = −log10x as the parent function, explain which transformation results in a y-intercept and why. You may graph by hand or using technology. Be sure to label each transformation on the graph. f(x) + 1 f(x + 1)

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 14:40
Chose the graph below that represents the following system of inequality’s
Answers: 2

Mathematics, 21.06.2019 16:50
Its worth 10000000 points need asap if you answer correctly ill mark brainliest
Answers: 1


Mathematics, 21.06.2019 18:00
If you had $1,900,000, how many days would it take you to spend all if you spent $1 a second. (there are 86,400 seconds in a day)
Answers: 1
You know the right answer?
Have you ever been swimming in a pool, and your eyes were red when you get out? This means the chemi...
Questions


Chemistry, 14.07.2019 03:10


Physics, 14.07.2019 03:10

Computers and Technology, 14.07.2019 03:10


Computers and Technology, 14.07.2019 03:10

Engineering, 14.07.2019 03:10

Computers and Technology, 14.07.2019 03:10

Computers and Technology, 14.07.2019 03:10

Computers and Technology, 14.07.2019 03:10

Mathematics, 14.07.2019 03:10



Mathematics, 14.07.2019 03:10

Computers and Technology, 14.07.2019 03:10


Computers and Technology, 14.07.2019 03:10