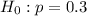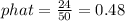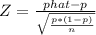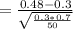Mathematics, 21.04.2020 00:52 lashayl27
Many investors and financial analysts believe the Dow Jones Industrial Average (DJIA) provides a good barometer of the overall stock market. On January 31, 2006, 9 of the 30 stocks making up the DJIA increased in price (The Wall Street Journal, February 1, 2006). On the basis of this fact, a financial analyst claims we can assume that 30% of the stocks traded on the New York Stock Exchange (NYSE) went up the same day.
1. Formulate null and alternative hypotheses to test the analyst's claim.
H0: p
Ha: p
2. A sample of 50 stocks traded on the NYSE that day showed that 24 went up. What is your point estimate of the population proportion of stocks that went up (to 2 decimals)?
3. Conduct your hypothesis test using = .01 as the level of significance.
Calculate the value of the test statistic (to 2 decimals).
What is the p-value (to 4 decimals)?
Can you conclude that the proportion of stocks going up is not .30?

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 20:40
What is the probability of throwing several dice with sum equal to 6 (six)? show the ways of possibilities for sum 6 (as the numerator) and the ways of throwing n dices for n = 1, 2, 3, 4 or 5 as denominator for all the questions to earn full credits. (a)throw one die, (b) throw two dice, (c) throw three dice (d) throw 4 dice, (e) throw 5 dice
Answers: 3

Mathematics, 21.06.2019 22:30
Will mark brainlist what is the slope of the line passing through the points (-2, -8) and (-3,-9)? -7/5-5/71-1
Answers: 2

Mathematics, 22.06.2019 03:10
Which of the following statements are true? (select all that apply.) a quasi-static process is one in which the system is never far from being in equilibrium. when a system can go from state 1 to state 2 by several different processes, the amount of heat absorbed by the system will be the same for all processes. the internal energy of a given amount of an ideal gas depends only on its absolute temperature. when a system can go from state 1 to state 2 by several different processes, the work done on the system will be the same for all processes. when a system can go from state 1 to state 2 by several different processes, the change in the internal energy of the system will be the same for all processes. for any substance that expands when heated, its cp is greater than its cv.
Answers: 2

Mathematics, 22.06.2019 03:50
Will mark brainliest, , and rate to only chrislaurencelle clarissa made a scale drawing of a rectangle. she used a scale factor of 3 to draw the new rectangle. how does the length of the new rectangle compare to the original? the length of the new rectangle is 3 times shorter than the original. the length of the new rectangle is 12 times shorter than the original. the length of the new rectangle is 3 times longer than the original. the length of the new rectangle is 12 times longer than the original.
Answers: 1
You know the right answer?
Many investors and financial analysts believe the Dow Jones Industrial Average (DJIA) provides a goo...
Questions



Engineering, 08.05.2021 14:00

Biology, 08.05.2021 14:00


Mathematics, 08.05.2021 14:00

Mathematics, 08.05.2021 14:00


English, 08.05.2021 14:00



English, 08.05.2021 14:00

Spanish, 08.05.2021 14:00

Mathematics, 08.05.2021 14:00

Mathematics, 08.05.2021 14:00

History, 08.05.2021 14:00


Business, 08.05.2021 14:00

Mathematics, 08.05.2021 14:00