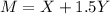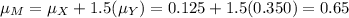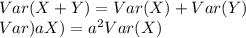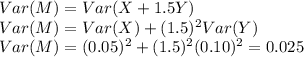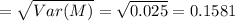Mathematics, 04.04.2020 10:54 Sbeech7362
The molarity of a solute in solution is defined to be the number of moles of solute per liter of solution (1 mole = 6.02 × 1023 molecules). If X is the molarity of a solution of magnesium chloride (MgCl2), and Y is the molarity of a solution of ferric chloride (FeCl3), the molarity of chloride ion (Cl−) in a solution made of equal parts of the solutions of MgCl2 and FeCl3 is given by M = X + 1.5Y . Assume that X has mean 0.125 and standard deviation 0.05, and that Y has mean 0.350 and standard deviation 0.10.
a. Find the mean of M
b. Find the standard deviation of M (assume X and Y are independent).
Please help, need accurate solutions

Answers: 2


Another question on Mathematics

Mathematics, 20.06.2019 18:04
Write each expression in exponential form and find its value.| ! 2 x 2 x 2 x 2
Answers: 1

Mathematics, 21.06.2019 18:30
Sasha drank 8 bottles of water everyday for a week. how many bottles of water did she drink in total?
Answers: 2

Mathematics, 21.06.2019 19:30
Ineed with angles and the measure of them i have abc a is 65 and b is (3x-10) and c is (2x) find the value of x
Answers: 2

Mathematics, 21.06.2019 21:00
Calculate the missing value. round the answer to on decimal place. start with 70, increase it by 21%, and end up with
Answers: 2
You know the right answer?
The molarity of a solute in solution is defined to be the number of moles of solute per liter of sol...
Questions

Mathematics, 02.11.2020 22:10

Mathematics, 02.11.2020 22:10

Social Studies, 02.11.2020 22:10

Advanced Placement (AP), 02.11.2020 22:10

Social Studies, 02.11.2020 22:10

Spanish, 02.11.2020 22:10


English, 02.11.2020 22:10



Biology, 02.11.2020 22:10


Mathematics, 02.11.2020 22:10

English, 02.11.2020 22:10

Computers and Technology, 02.11.2020 22:10


Mathematics, 02.11.2020 22:10

Mathematics, 02.11.2020 22:10

Biology, 02.11.2020 22:10