Mathematics, 26.03.2020 16:36 mmaglaya1
The volume of an ideal gas is increased from 0.61 m3 to 3.9 m3 while maintaining a constant pressure of 970 Pa (1 Pa = 1 N/m2). If the initial temperature of the gas is 65 K, (a) What is the final temperature of the gas?

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 17:20
Read the equations in the table below. then drag a description of the situation and a table to represent each equation. indicate whether each of the relationships is proportional or non-proportional.
Answers: 1

Mathematics, 21.06.2019 18:10
The number of branches on a tree demonstrates the fibonacci sequence. how many branches would there be on the next two levels of this tree? 13 | | | m branches
Answers: 3

Mathematics, 21.06.2019 18:30
In the following diagram it is given that dec,ab || dc, ad || eb, and ad is congruent to bc. a)why isn't abc a parallelogram even though it has a pair of parallel sides and a pair of congruent sides b)explain why be must be congruent to bc. further explain what type of triangle this makes triangle abc and what it tells you about angle 1 and angle 2c) finally why must angle 3 be congruent to angle 1? further, explain why we know that angle 3 is congruent to angle 2
Answers: 1

You know the right answer?
The volume of an ideal gas is increased from 0.61 m3 to 3.9 m3 while maintaining a constant pressure...
Questions

Mathematics, 18.03.2021 01:50




Mathematics, 18.03.2021 01:50





History, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50



Spanish, 18.03.2021 01:50




Mathematics, 18.03.2021 01:50

Social Studies, 18.03.2021 01:50

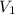 = 0.61 m^3 => 610L
= 0.61 m^3 => 610L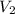 = 3.9 m^3 => 3900L
= 3.9 m^3 => 3900L 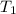 = 65 K
= 65 K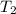 =?
=?