Mathematics, 27.02.2020 19:26 nickwwe13
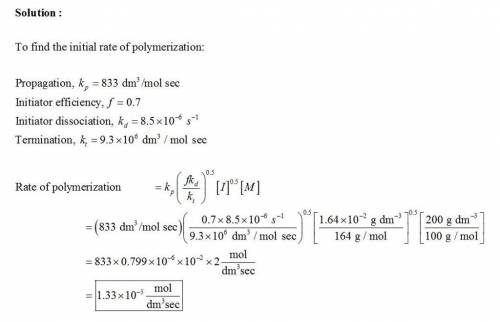
Methyl methacrylate was polymerized at a mass concentration of 200 g/dm3 in toluene using azobisisobutyronitrile (AIBN) as initiator at a mass concentration of 1.64 x 10-2 g/dm3 and a reaction temperature of 60°C. Calculate the initial rate of polymerization and the number average molar mass of poly(methyl methacrylate) formed in the initial stages of the reaction given that the relevant rate coefficients at 60°C are:
Initiator dissociation, kd = 8.5 x 10-6 s-1
Propagation, kp = 833 dm3 mol-1 s-1
Termination, kt = 9.3 x 106 dm3 mol-1 s-1
Transfer to monomer, ktrM = 3.93 x 10-3 dm3 mol-1 s-1
Transfer to solvent, ktrS = 7.34 x 10-3 dm3 mol-1 s-1
Initiator efficiency, f = 0.7 Termination by combination is negligible Density of initial solution of methyl methacrylate in toluene is 860 g/dm3

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 18:30
To determine the number of deer in a forest a forest ranger tags 280 and releases them back
Answers: 3

Mathematics, 21.06.2019 18:50
The trigonometric ratios sine and secant are reciprocals of each other
Answers: 2

Mathematics, 21.06.2019 22:00
Using inductive reasoning, what is the next two numbers in this set? 1,-7,13,-19 i got the numbers 14,-26 is that right?
Answers: 2

Mathematics, 21.06.2019 22:30
Nicole is making carrot bread she needs 5 cups of carrots for 2 cups of sugar.what is the ratio of cups of sugar to cups of carrots that nicole will need? write the ration in three different ways
Answers: 1
You know the right answer?
Methyl methacrylate was polymerized at a mass concentration of 200 g/dm3 in toluene using azobisisob...
Questions



History, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01




Mathematics, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01


Biology, 06.10.2020 14:01


Physics, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01