Mathematics, 25.02.2020 21:55 send2tejas
The decomposition of HI(g) at 298K is represented by the equilibrium equation above. When 100.torr of HI(g) is added to a previously evacuated, rigid container and allowed to reach equilibrium, the partial pressure of I2(g) is approximately 3.7torr. If the initial pressure of HI(g) is increased to 200.torr and the process is repeated at the same temperature, which of the following correctly predicts the equilibrium partial pressure of I2(g), and why

Answers: 3


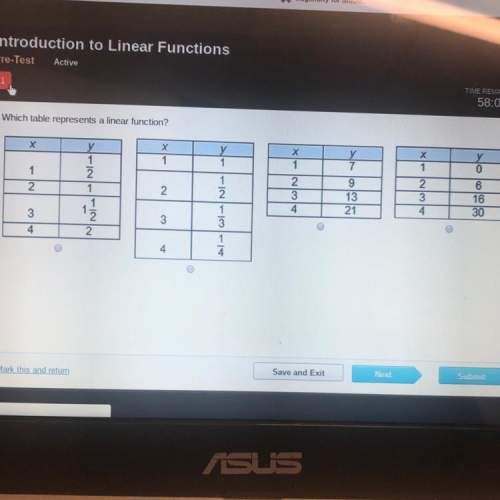
Another question on Mathematics

Mathematics, 21.06.2019 20:30
Secant be and cf intersect at point d inside a what is the measure of cde
Answers: 1

Mathematics, 21.06.2019 22:20
The coordinates of vortex a’ are (8,4) (-4,2) (4,-,4) the coordinates of vortex b’ are (6,6)(12,-,12)(12,12) the coordinates of vortex c’ are(-2,,12)(4,12)(12,-4)
Answers: 3

Mathematics, 21.06.2019 22:20
Line segment eg is partitioned by point f in the ratio 1: 1. point e is at e (0, 4), and point f is at (1, 3). what are the coordinates of point g? (−1, 5) (2, 2) (3, 1) (4, 0)
Answers: 2

Mathematics, 22.06.2019 02:30
In the next 10 month,colin wants to save $900 for his vacation.he plans to save $75 each of the first 8 months. how much must he save each of the last 2 months in order to meet his goal if he saves the same amount each month ?
Answers: 1
You know the right answer?
The decomposition of HI(g) at 298K is represented by the equilibrium equation above. When 100.torr o...
Questions



Mathematics, 19.07.2019 10:20

Biology, 19.07.2019 10:20

English, 19.07.2019 10:20






Mathematics, 19.07.2019 10:30

Mathematics, 19.07.2019 10:30

Social Studies, 19.07.2019 10:30





Mathematics, 19.07.2019 10:30


Physics, 19.07.2019 10:30