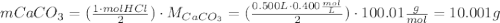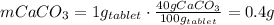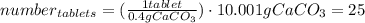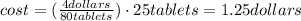Mathematics, 23.11.2019 02:31 davisdarby2
One container of tums® costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tums® is 40.0 percent caco₃ by mass. using only tums®, you are required to neutralize 0.500 l of 0.400 m hcl. how much does this cost? assume you are able to purchase individual tablets. express your answer in dollars.

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 15:00
Consider the diagram. which line segment has the same measure as st? rx tx sr xs
Answers: 3


Mathematics, 21.06.2019 21:10
Which exponential function has an initial value of 2? f(x) = 2(3x) f(x) = 3(2x)
Answers: 1

Mathematics, 22.06.2019 00:40
The point (-7, -24) is on the terminal ray of angle 0 which is in standard position. a student found the six trigonometric values for angle e. the student's answers are shown. which value(s) are incorrect? sin(8) cos(8) 24 tan(0) sin(0)=25 cos(0) -- tan(ⓡ) - - 24 csc(o)=2 sec(0) --25 cot(6) - za csc(o) sec(0) cot(0) done
Answers: 3
You know the right answer?
One container of tums® costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tum...
Questions






History, 02.12.2019 00:31

Mathematics, 02.12.2019 00:31

Mathematics, 02.12.2019 00:31

Mathematics, 02.12.2019 00:31



Physics, 02.12.2019 00:31



Biology, 02.12.2019 00:31

Biology, 02.12.2019 00:31

Mathematics, 02.12.2019 00:31

Mathematics, 02.12.2019 00:31


Health, 02.12.2019 00:31