
Mathematics, 01.08.2019 05:10 kactus
The standard cell potential (e°) of a voltaic cell constructed using the cell reaction below is 0.76 v: zn (s) + 2h+ (aq) → zn2+ (aq) + h2 (g) with ph2 = 1.0 atm and [zn2+] = 1.0 m, the cell potential is 0.52 v. the concentration of h+ in the cathode compartment is m.

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 13:30
Combined megan and kelly worked 60 hours.kelly worked twice as many hours as megan.how many hours did they each worked?
Answers: 3

Mathematics, 21.06.2019 22:00
Ascientist has 50 grams of a radioactive element. the amount of radioactive element remaining after t days can be determined using the equation (1) after two days the scientist receives a second shipment of 50 grams of the same element. the equation used to represent the amount of shipment 2 remaining after t days is 10) - 50 which of the following is an equivalent form of the expression for the amount remaining in shipment 2? what’s the answer?
Answers: 2

Mathematics, 21.06.2019 22:00
Given that sin∅ = 1/4, 0 < ∅ < π/2, what is the exact value of cos∅? a. (√4)/4 b. (√15)/4 c. (4π)/2 d. (4√2)/4
Answers: 2

Mathematics, 22.06.2019 01:30
Im so bad at fractions they are not my best math thing to work on
Answers: 3
You know the right answer?
The standard cell potential (e°) of a voltaic cell constructed using the cell reaction below is 0.76...
Questions

Mathematics, 14.10.2019 08:50


Business, 14.10.2019 08:50



History, 14.10.2019 08:50


English, 14.10.2019 08:50



Mathematics, 14.10.2019 08:50



History, 14.10.2019 08:50



Mathematics, 14.10.2019 09:00

Mathematics, 14.10.2019 09:00

Mathematics, 14.10.2019 09:00

English, 14.10.2019 09:00

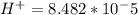 M
M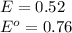
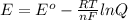
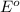 is the standard cell potential
is the standard cell potential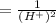
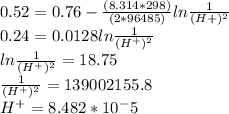 M
M

